Bacterial chemoreceptors: high-performance signaling in networked arrays
- PMID: 18165013
- PMCID: PMC2890293
- DOI: 10.1016/j.tibs.2007.09.014
Bacterial chemoreceptors: high-performance signaling in networked arrays
Abstract
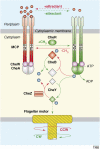
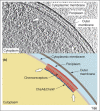
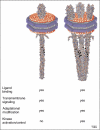
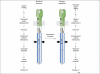
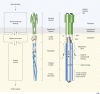
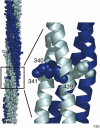
Chemoreceptors are crucial components in the bacterial sensory systems that mediate chemotaxis. Chemotactic responses exhibit exquisite sensitivity, extensive dynamic range and precise adaptation. The mechanisms that mediate these high-performance functions involve not only actions of individual proteins but also interactions among clusters of components, localized in extensive patches of thousands of molecules. Recently, these patches have been imaged in native cells, important features of chemoreceptor structure and on-off switching have been identified, and new insights have been gained into the structural basis and functional consequences of higher order interactions among sensory components. These new data suggest multiple levels of molecular interactions, each of which contribute specific functional features and together create a sophisticated signaling device.
Figures
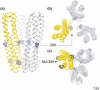
References
-
- Bourret RB, Stock AM. Molecular information processing: lessons from bacterial chemotaxis. J. Biol. Chem. 2002;277:9625–9628. - PubMed
-
- Sourjik V. Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol. 2004;12:569–576. - PubMed
-
- Parkinson JS, et al. Collaborative signaling by bacterial chemoreceptors. Curr. Opin. Microbiol. 2005;8:116–121. - PubMed
-
- Baker MD, et al. Systems biology of bacterial chemotaxis. Curr. Opin. Microbiol. 2006;9:187–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

