The type IV secretion system component VirB5 binds to the trans-zeatin biosynthetic enzyme Tzs and enables its translocation to the cell surface of Agrobacterium tumefaciens
- PMID: 18165307
- PMCID: PMC2258690
- DOI: 10.1128/JB.01718-07
The type IV secretion system component VirB5 binds to the trans-zeatin biosynthetic enzyme Tzs and enables its translocation to the cell surface of Agrobacterium tumefaciens
Abstract
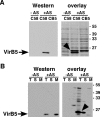
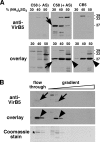
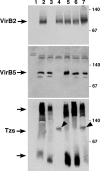
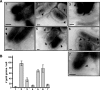
VirB5 is a minor component of the extracellular T pilus determined by the Agrobacterium tumefaciens type IV secretion system. To identify proteins that interact with VirB5 during the pilus assembly process, we purified VirB5 as a recombinant fusion protein and, by using a gel overlay assay, we detected a 26-kDa interacting protein in Agrobacterium cell lysates. The VirB5-binding protein was purified from A. tumefaciens and identified as the cytokinin biosynthetic enzyme Tzs. The VirB5-Tzs interaction was confirmed using pulldown assays with purified proteins and the yeast two-hybrid system. An analysis of the subcellular localization in A. tumefaciens showed that Tzs was present in the soluble as well as the membrane fraction. Tzs was extracted from the membranes with the mild detergent dodecyl-beta-D-maltoside in complexes of different molecular masses, and this association was strongly reduced in the absence of VirB5. Using immunoelectron microscopy, we also detected Tzs on the Agrobacterium cell surface. A functional type IV secretion system was required for efficient translocation to the surface, but Tzs was not secreted into the cell supernatant. The fact that Tzs localizes on the cell surface suggests that it may contribute to the interaction of Agrobacterium with plants.
Figures



References
-
- Aly, K. A., and C. Baron. 2007. The VirB5 protein localizes to the T-pilus tips in Agrobacterium tumefaciens. Microbiology 1533766-3775. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

