The genetic design of signaling cascades to record receptor activation
- PMID: 18165312
- PMCID: PMC2224232
- DOI: 10.1073/pnas.0710487105
The genetic design of signaling cascades to record receptor activation
Abstract
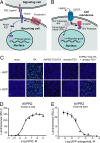
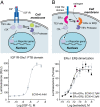
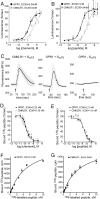
We have developed an experimental strategy to monitor protein interactions in a cell with a high degree of selectivity and sensitivity. A transcription factor is tethered to a membrane-bound receptor with a linker that contains a cleavage site for a specific protease. Activation of the receptor recruits a signaling protein fused to the protease that then cleaves and releases the transcription factor to activate reporter genes in the nucleus. This strategy converts a transient interaction into a stable and amplifiable reporter gene signal to record the activation of a receptor without interference from endogenous signaling pathways. We have developed this assay for three classes of receptors: G protein-coupled receptors, receptor tyrosine kinases, and steroid hormone receptors. Finally, we use the assay to identify a ligand for the orphan receptor GPR1, suggesting a role for this receptor in the regulation of inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wehr MC, Laage R, Bolz U, Fischer TM, Grunewald S, et al. Monitoring regulated protein-protein interactions using split TEV. Nat Methods. 2006;3:985–993. - PubMed
-
- Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–660. - PubMed
-
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. - PubMed
-
- Parks TD, Leuther KK, Howard ED, Johnston SA, Dougherty WG. Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Anal Biochem. 1994;216:413–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

