Marker removal in staphylococci via Cre recombinase and different lox sites
- PMID: 18165371
- PMCID: PMC2258651
- DOI: 10.1128/AEM.02424-07
Marker removal in staphylococci via Cre recombinase and different lox sites
Abstract
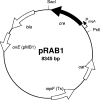
Allelic replacement in staphylococci is frequently aided by antibiotic resistance markers that replace the gene(s) of interest. In multiply modified strains, the number of mutated genes usually correlates with the number of selection markers in the strain's chromosome. Site-specific recombination systems are capable of eliminating such markers, if they are flanked by recombinase recognition sites. In this study, a Cre-lox setting was established that allowed the efficient removal of resistance genes from the genomes of Staphylococcus carnosus and S. aureus. Two cassettes conferring resistance to erythromycin or kanamycin were flanked with wild-type or mutant lox sites, respectively, and used to delete single genes and an entire operon. After transformation of the cells with a newly constructed cre expression plasmid (pRAB1), genomic eviction of the resistance genes was observed in approximately one out of ten candidates analyzed and subsequently verified by PCR. Due to its thermosensitive origin of replication, the plasmid was then easily eliminated at nonpermissive temperatures. We anticipate that the system presented here will prove useful for generating markerless deletion mutants in staphylococci.
Figures




References
-
- Albert, H., E. C. Dale, E. Lee, and D. W. Ow. 1995. Site-specific integration of DNA into wild-type and mutant lox sites placed in the plant genome. Plant J. 7:649-659. - PubMed
-
- Augustin, J., and F. Götz. 1990. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol. Lett. 54:203-207. - PubMed
-
- Ayres, E. K., V. J. Thomson, G. Merino, D. Balderes, and D. H. Figurski. 1993. Precise deletions in large bacterial genomes by vector-mediated excision (VEX): the trfA gene of promiscuous plasmid RK2 is essential for replication in several gram-negative hosts. J. Mol. Biol. 230:174-185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

