A single-molecule nanopore device detects DNA polymerase activity with single-nucleotide resolution
- PMID: 18166054
- PMCID: PMC2453067
- DOI: 10.1021/ja077082c
A single-molecule nanopore device detects DNA polymerase activity with single-nucleotide resolution
Abstract
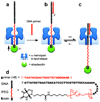
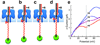
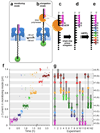
The ability to monitor DNA polymerase activity with single-nucleotide resolution has been the cornerstone of a number of advanced single-molecule DNA sequencing concepts. Toward this goal, we report the first observation of the base-by-base DNA polymerase activity with single-base resolution at the single-molecule level. We describe the design and characterization of a supramolecular nanopore device capable of detecting up to nine consecutive DNA polymerase-catalyzed single-nucleotide primer extensions with high sensitivity and spatial resolution (<or=2.4 A). The device is assembled in a suspended lipid membrane by threading and mechanically capturing a single strand of DNA-PEG copolymer inside an alpha-hemolysin protein pore. Single-nucleotide primer extensions result in successive displacements of the template DNA strand within the protein pore, which can be monitored by the corresponding stepped changes in the ion current flowing through the pore under an applied transmembrane potential. The system described thus represents a promising advance toward nanopore-mediated single-molecule DNA sequencing concept and, in addition, might be applicable to studying a number of other biopolymer-protein interactions and dynamics.
Figures
References
-
- Maier B, Bensimon D, Croquette V. Proc. Natl. Acad. Sci. USA. 2000;97:12002–12007. - PMC - PubMed
- Braslavsky I, Hebert B, Kartalov E, Quake SR. Proc. Natl. Acad. Sci. USA. 2003;100:3960–3964. - PMC - PubMed
- Bustamante C, Bryant Z, Smith SB. Nature. 2003;421:423–427. - PubMed
- Abbondanzieri EA, Greenleaf WJ, Shaevitz JW, Landick R, Block SM. Nature. 2005;438:460–465. - PMC - PubMed
- Greenleaf WJ, Block SM. Science. 2006;313:801. - PMC - PubMed
-
- Sanchez-Quesada J, Saghatelian A, Cheley S, Bayley H, Ghadiri MR. Angew. Chem. Int. Ed. 2004;43:3063–3067. - PMC - PubMed
- Mathe J, Visram H, Viasnoff V, Rabin Y, Meller A. Biophys. J. 2004;87:3205–3212. - PMC - PubMed
- Nakane J, Wiggin M, Marziali A. Biophys. J. 2004;87:615–621. - PMC - PubMed
- Ashkenasy N, Sanchez-Quesada J, Bayley H, Ghadiri MR. Angew. Chem. Int. Ed. 2005;44:1401–1404. - PMC - PubMed
-
- Kasianowicz JJ, Brandin E, Branton D, Deamer DW. Proc. Natl. Acad. Sci. USA. 1996;93:13770–13773. - PMC - PubMed
- Vercoutere W, Winters-Hilt S, Olsen H, Deamer DW, Haussler D, Akeson M. Nat. Biotechnol. 2001;19:248–252. - PubMed
- Rhee M, Burns MA. Trends Biotechnol. 2006;24:580–586. - PubMed
- Astier Y, Braha O, Bayley H. J. Am. Chem. Soc. 2006;128:1705–1710. - PubMed
-
- Movileanu L, Howorka S, Braha O, Bayley H. Nat. Biotechnol. 2000;18:1091–1095. - PubMed
- Howorka S, Cheley S, Bayley H. Nat. Biotechnol. 2001;19:636–639. - PubMed
- Cheley S, Xie H, Bayley H. ChemBioChem. 2006;7:1923–1927. - PubMed
- Hornblower B, Coombs A, Whitaker RD, Kolomeisky A, Picone SJ, Meller A, Akeson M. Nat. Methods. 2007;4:315–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

