Progression of Plasmodium berghei through Anopheles stephensi is density-dependent
- PMID: 18166078
- PMCID: PMC2156095
- DOI: 10.1371/journal.ppat.0030195
Progression of Plasmodium berghei through Anopheles stephensi is density-dependent
Abstract
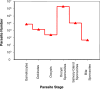
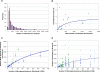
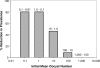
It is well documented that the density of Plasmodium in its vertebrate host modulates the physiological response induced; this in turn regulates parasite survival and transmission. It is less clear that parasite density in the mosquito regulates survival and transmission of this important pathogen. Numerous studies have described conversion rates of Plasmodium from one life stage to the next within the mosquito, yet few have considered that these rates might vary with parasite density. Here we establish infections with defined numbers of the rodent malaria parasite Plasmodium berghei to examine how parasite density at each stage of development (gametocytes; ookinetes; oocysts and sporozoites) influences development to the ensuing stage in Anopheles stephensi, and thus the delivery of infectious sporozoites to the vertebrate host. We show that every developmental transition exhibits strong density dependence, with numbers of the ensuing stages saturating at high density. We further show that when fed ookinetes at very low densities, oocyst development is facilitated by increasing ookinete number (i.e., the efficiency of ookinete-oocyst transformation follows a sigmoid relationship). We discuss how observations on this model system generate important hypotheses for the understanding of malaria biology, and how these might guide the rational analysis of interventions against the transmission of the malaria parasites of humans by their diverse vector species.
Conflict of interest statement
Figures
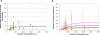


References
-
- Vaughan JA. Population dynamics of Plasmodium sporogony. Trends Parasitol. 2007;23:63–70. - PubMed
-
- Pringle GA. Count of the sporozoites in an oocyst of Plasmodium falciparum . Trans R Soc Trop Med Hyg. 1965;59:289–290. - PubMed
-
- Rosenberg R, Rungiwongse J. The number of sporozoites produced by individual malaria oocysts. Am J Trop Med Hyg. 1991;45:574–577. - PubMed
-
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. - PubMed
-
- Frischknecht F, Baldacci P, Martin B, Zimmer C, Thiberge S. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell Microbiol. 2004;6:687–694. - PubMed

