Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia
- PMID: 18166354
- PMCID: PMC2654267
- DOI: 10.1053/j.gastro.2007.10.002
Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia
Abstract
Background & aims: Eosinophilic esophagitis (EE) is an increasingly recognized disease that mimics gastroesophageal reflux disease. Recently, EE has been associated with esophageal remodeling, but the mechanisms involved are poorly understood. We hypothesized that the development of EE in patients and in an experimental murine model would be associated with eosinophil-mediated tissue remodeling.
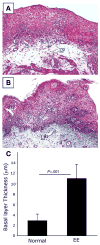
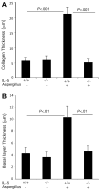
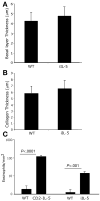
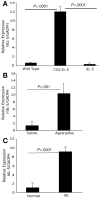
Methods: Histopathologic analysis of basal layer thickness and collagen accumulation was performed on the biopsy specimens of normal individuals, EE patients, and mouse esophageal tissue sections following experimental induction of EE in wild-type, eosinophil lineage-deficient, interleukin (IL)-5-deficient, and IL-5 transgenic mice, with the latter 2 mice groups having decreased and increased esophageal eosinophilia, respectively.
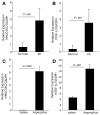
Results: An impressive accumulation of collagen in the epithelial mucosa and lamina propria, as well as basal layer thickening, was observed in the esophagus of patients with EE as well as in mice with experimental EE compared with controls. Significantly reduced lamina propria collagen and basal layer thickness were observed in IL-5-deficient mice and eosinophil lineage-deficient mice compared with wild-type mice following the induction of experimental EE. Furthermore, the esophagus of CD2-IL-5 transgenic mice showed increased basal layer thickness and collagen accumulation compared with nontransgenic mice, yet IL-5 intestine transgenic mice did not have EE-like esophageal changes. Additional analysis revealed increased IL-5 levels in the esophagus of EE patients, allergen-challenged wild-type mice, and CD2-IL-5 transgenic mice but not in IL-5 intestine transgenic mice.
Conclusions: These findings provide evidence that local IL-5-mediated eosinophilia is essential in the induction of esophageal remodeling.
Figures



References
-
- Bousquet J, Jeffery PK, Busse WW, et al. Asthma. From broncho-constriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. - PubMed
-
- Jeffery PK, Wardlaw AJ, Nelson FC, et al. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–1753. - PubMed
-
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. - PubMed
-
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–S38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

