Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial
- PMID: 18167062
- PMCID: PMC3755275
- DOI: 10.1002/hep.22094
Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial
Abstract
There is relatively little information in the literature on the histopathology of chronic hepatitis C in children. The Peds-C Trial, designed to test the efficacy and safety of peginterferon alfa-2a and ribavirin in children, provided an opportunity to examine liver biopsies from 121 treatment-naïve children, ages 2 to 16 (mean, 9.8 years) infected with the hepatitis C virus (HCV) and with no other identifiable cause for liver disease, signs of hepatic decompensation, or another significant nonhepatic disease. Liver biopsies were scored for inflammation, fibrosis, steatosis, and other histological features. Inflammation in the biopsy was minimal in 42%, mild in 17%, moderate in 38%, and severe in only 3%. Five had bridging fibrosis, and 2 had cirrhosis. Steatosis was absent in 56%, minimal in 34%, and mild in 10%. Inflammation scores correlated with fibrosis scores, serum alanine aminotransferase levels, and duration of infection, but not with age, body mass index z score, or HCV genotype. Fibrosis scores correlated with inflammation but not with age, HCV genotype, body mass index z score, or steatosis parameters. Steatosis correlated with serum alanine aminotransferase levels and body mass index z scores; overweight children had more fibrosis than the non-overweight. In conclusion, in this cohort of HCV-infected children, inflammation, fibrosis, and steatosis were milder than reported for treatment-naïve adults with chronic hepatitis C, but there were several with bridging fibrosis or cirrhosis. The positive correlation of inflammation with duration of infection and fibrosis and of obesity with fibrosis suggest that children with chronic hepatitis C will be at risk for progressive liver disease as they age and possibly acquire other comorbid risk factors.
Conflict of interest statement
Potential conflict of interest: Dr. Mohan received grants from Gilead and Roche.
Figures
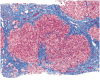
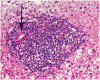

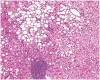
Comment in
-
Children with chronic hepatitis C: what future?Hepatology. 2008 Aug;48(2):691-2; author reply 692. doi: 10.1002/hep.22389. Hepatology. 2008. PMID: 18666239 No abstract available.
References
-
- Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. - PubMed
-
- Goodman ZD, Ishak KG. Histopathology of hepatitis C virus infection. Semin Liver Dis. 1995;15:70–81. - PubMed
-
- Dhillon AP, Dusheiko GM. Pathology of hepatitis C virus infection. Histopathology. 1995;26:297–309. - PubMed
-
- Kage M, Fujisawa T, Shiraki K, Tanaka T, Fujisawa T, Kimura A, Shimamatsu K, et al. Pathology of chronic hepatitis C in children. Hepatology. 1997;26:771–775. - PubMed
-
- Badizadegan K, Jonas MM, Ott MJ, Nelson SP, Perez-Atayde AR. Histopathology of the liver in children with chronic hepatitis C viral infection. Hepatology. 1998;28:1416–1423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
