Variability and action mechanism of a family of anticomplement proteins in Ixodes ricinus
- PMID: 18167559
- PMCID: PMC2151134
- DOI: 10.1371/journal.pone.0001400
Variability and action mechanism of a family of anticomplement proteins in Ixodes ricinus
Abstract
Background: Ticks are blood feeding arachnids that characteristically take a long blood meal. They must therefore counteract host defence mechanisms such as hemostasis, inflammation and the immune response. This is achieved by expressing batteries of salivary proteins coded by multigene families.
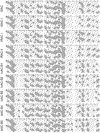
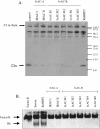
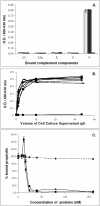
Methodology/principal findings: We report the in-depth analysis of a tick multigene family and describe five new anticomplement proteins in Ixodes ricinus. Compared to previously described Ixodes anticomplement proteins, these segregated into a new phylogenetic group or subfamily. These proteins have a novel action mechanism as they specifically bind to properdin, leading to the inhibition of C3 convertase and the alternative complement pathway. An excess of non-synonymous over synonymous changes indicated that coding sequences had undergone diversifying selection. Diversification was not associated with structural, biochemical or functional diversity, adaptation to host species or stage specificity but rather to differences in antigenicity.
Conclusions/significance: Anticomplement proteins from I. ricinus are the first inhibitors that specifically target a positive regulator of complement, properdin. They may provide new tools for the investigation of role of properdin in physiological and pathophysiological mechanisms. They may also be useful in disorders affecting the alternative complement pathway. Looking for and detecting the different selection pressures involved will help in understanding the evolution of multigene families and hematophagy in arthropods.
Conflict of interest statement
Figures

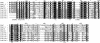





References
-
- Price PW. Princeton, New Jersey: Princeton University Press; 1980. Evolutionary evolutionary biology of parasites. Monographs in population biology. p. 237. - PubMed
-
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. - PubMed
-
- Sonenshine DE. New York, Oxford: Oxford University Press; 1991. Biology of ticks. Vol. I.
-
- Mans BJ, Gothe R, Neitz AWH. Biochemical perspectives on paralysis and other forms of toxicoses caused by ticks. Parasitology. 2004;129:S95–S111. - PubMed
-
- Ribeiro JMC. Role of saliva in blood-feeding by arthropods. Ann Rev Entomol. 1987a;32:463–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

