Outer segment oligomerization of Rds: evidence from mouse models and subcellular fractionation
- PMID: 18171083
- PMCID: PMC2788309
- DOI: 10.1021/bi701807c
Outer segment oligomerization of Rds: evidence from mouse models and subcellular fractionation
Abstract
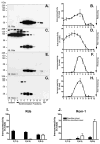
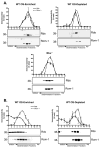
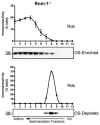
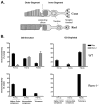
Retinal degeneration slow (Rds) is a photoreceptor-specific tetraspanin glycoprotein essential for photoreceptor outer segment (OS) morphogenesis. Over 80 mutations in this protein are associated with several different retinal diseases. Rds forms a mixture of disulfide-linked homomeric dimers, octamers, and higher-order oligomers, with Cys150 playing a crucial role in its oligomerization. Rds also forms noncovalent homo- and hetero-tetramers with its nonglycosylated homologue, Rom-1. Here, we evaluated the subcellular site of Rds oligomerization and the pattern of Rds/Rom-1 complex assembly in several types of knockout mice, including rhodopsin (Rho-/-, lacking rod OS), Rom-1 (Rom-1-/-), neural retina leucine zipper (Nrl-/-, cone-dominant), and in comparison with wild-type (WT, rod-dominant) mice. Oligomerization and the pattern of complex assembly were also evaluated in OS-enriched vs OS-depleted preparations from WT and Rom-1-/- retinas. Velocity sedimentation under reducing- and nonreducing conditions and co-immunoprecipitation experiments showed the presence of Rds mainly as homo- and hetero-tetramers with Rom-1 in the photoreceptor inner segment (IS), while higher-order, disulfide-linked intermediate complexes and oligomers were exclusively present in the photoreceptor OS. Rom-1-independent oligomerization of Rds was observed in Rom-1-/- retinas. The pattern of Rds complexes in cones from Nrl-/- mice was comparable to that in rods from WT mice. On the basis of these findings, we propose that Rds traffics from the IS to the OS as homo- and hetero-tetramers, with subsequent disulfide-linked oligomerization occurring concomitant with OS disc morphogenesis (at either the base of OS or the tip of the connecting cilium). These results suggest that Rds mutations that interfere with tetramer formation can block Rds trafficking to the OS, leading to loss-of-function defects.
Figures



References
-
- Molday RS, Hicks D, Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987;28:50–61. - PubMed
-
- Bascom RA, Manara S, Collins L, Molday RS, Kalnins VI, McInnes RR. Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron. 1992;8:1171–1184. - PubMed
-
- Sanyal S, Jansen HG. Absence of receptor outer segments in the retina of Rds mutant mice. Neurosci Lett. 1981;21:23–26. - PubMed
-
- Travis GH, Sutcliffe JG, Bok D. The retinal degeneration slow (Rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991;6:61–70. - PubMed
-
- Kajiwara K, Hahn LB, Mukai S, Travis GH, Berson EL, Dryja TP. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991;354:480–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

