Amiloride-sensitive channels in type I fungiform taste cells in mouse
- PMID: 18171468
- PMCID: PMC2235881
- DOI: 10.1186/1471-2202-9-1
Amiloride-sensitive channels in type I fungiform taste cells in mouse
Abstract
Background: Taste buds are the sensory organs of taste perception. Three types of taste cells have been described. Type I cells have voltage-gated outward currents, but lack voltage-gated inward currents. These cells have been presumed to play only a support role in the taste bud. Type II cells have voltage-gated Na+ and K+ current, and the receptors and transduction machinery for bitter, sweet, and umami taste stimuli. Type III cells have voltage-gated Na+, K+, and Ca2+ currents, and make prominent synapses with afferent nerve fibers. Na+ salt transduction in part involves amiloride-sensitive epithelial sodium channels (ENaCs). In rodents, these channels are located in taste cells of fungiform papillae on the anterior part of the tongue innervated by the chorda tympani nerve. However, the taste cell type that expresses ENaCs is not known. This study used whole cell recordings of single fungiform taste cells of transgenic mice expressing GFP in Type II taste cells to identify the taste cells responding to amiloride. We also used immunocytochemistry to further define and compare cell types in fungiform and circumvallate taste buds of these mice.
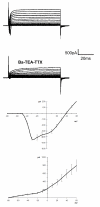
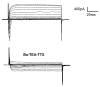
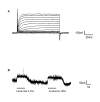
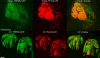
Results: Taste cell types were identified by their response to depolarizing voltage steps and their presence or absence of GFP fluorescence. TRPM5-GFP taste cells expressed large voltage-gated Na+ and K+ currents, but lacked voltage-gated Ca2+ currents, as expected from previous studies. Approximately half of the unlabeled cells had similar membrane properties, suggesting they comprise a separate population of Type II cells. The other half expressed voltage-gated outward currents only, typical of Type I cells. A single taste cell had voltage-gated Ca2+ current characteristic of Type III cells. Responses to amiloride occurred only in cells that lacked voltage-gated inward currents. Immunocytochemistry showed that fungiform taste buds have significantly fewer Type II cells expressing PLC signalling components, and significantly fewer Type III cells than circumvallate taste buds.
Conclusion: The principal finding is that amiloride-sensitive Na+ channels appear to be expressed in cells that lack voltage-gated inward currents, likely the Type I taste cells. These cells were previously assumed to provide only a support function in the taste bud.
Figures


References
-
- Finger TE., Simon, S.A. Cell Biology of Taste Epithelium. In Neurobiology of Taste and Smell, 2nd edition Edited by Finger, TE, Silver, WL, Restrepo, D New York: Wiley Liss. 2000. pp. 287–314.
-
- Kinnamon SC., Margolskee, R.F. Taste Transduction. In Handbook of Senses Volume 1 Edited by Firestein, S, Smith, D, Shepherd, G In Press. 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

