Structures of open (R) and close (T) states of prephenate dehydratase (PDT)--implication of allosteric regulation by L-phenylalanine
- PMID: 18171624
- PMCID: PMC3366492
- DOI: 10.1016/j.jsb.2007.11.009
Structures of open (R) and close (T) states of prephenate dehydratase (PDT)--implication of allosteric regulation by L-phenylalanine
Abstract
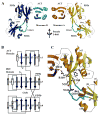
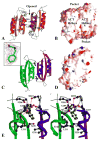
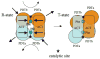
The enzyme prephenate dehydratase (PDT) converts prephenate to phenylpyruvate in L-phenylalanine biosynthesis. PDT is allosterically regulated by L-Phe and other amino acids. We report the first crystal structures of PDT from Staphylococcus aureus in a relaxed (R) state and PDT from Chlorobium tepidum in a tense (T) state. The two enzymes show low sequence identity (27.3%) but the same prototypic architecture and domain organization. Both enzymes are tetramers (dimer of dimers) in crystal and solution while a PDT dimer can be regarded as a basic catalytic unit. The N-terminal PDT domain consists of two similar subdomains with a cleft in between, which hosts the highly conserved active site. In one PDT dimer two clefts are aligned to form an extended active site across the dimer interface. Similarly at the interface two ACT regulatory domains create two highly conserved pockets. Upon binding of the L-Phe inside the pockets, PDT transits from an open to a closed conformation.
Figures


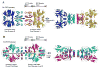

Similar articles
-
X-ray structure of prephenate dehydratase from Streptococcus mutans.J Microbiol. 2014 Jun;52(6):490-5. doi: 10.1007/s12275-014-3645-8. Epub 2014 Mar 7. J Microbiol. 2014. PMID: 24610334
-
Probing the catalytic mechanism of prephenate dehydratase by site-directed mutagenesis of the Escherichia coli P-protein dehydratase domain.Biochemistry. 2000 Apr 25;39(16):4722-8. doi: 10.1021/bi9926680. Biochemistry. 2000. PMID: 10769128
-
pheA (Rv3838c) of Mycobacterium tuberculosis encodes an allosterically regulated monofunctional prephenate dehydratase that requires both catalytic and regulatory domains for optimum activity.J Biol Chem. 2005 May 27;280(21):20666-71. doi: 10.1074/jbc.M502107200. Epub 2005 Mar 7. J Biol Chem. 2005. PMID: 15753077
-
Prephenate dehydratase of the actinomycete Amycolatopsis methanolica: purification and characterization of wild-type and deregulated mutant proteins.Biochem J. 1995 May 15;308 ( Pt 1)(Pt 1):313-20. doi: 10.1042/bj3080313. Biochem J. 1995. PMID: 7755580 Free PMC article.
-
Allosteric mechanisms in ACT domain containing enzymes involved in amino acid metabolism.Amino Acids. 2005 Feb;28(1):1-12. doi: 10.1007/s00726-004-0152-y. Epub 2005 Jan 18. Amino Acids. 2005. PMID: 15662561 Review.
Cited by
-
Domain Movements upon Activation of Phenylalanine Hydroxylase Characterized by Crystallography and Chromatography-Coupled Small-Angle X-ray Scattering.J Am Chem Soc. 2016 May 25;138(20):6506-16. doi: 10.1021/jacs.6b01563. Epub 2016 May 12. J Am Chem Soc. 2016. PMID: 27145334 Free PMC article.
-
Characterization of two key enzymes for aromatic amino acid biosynthesis in symbiotic archaea.Extremophiles. 2016 Jul;20(4):503-14. doi: 10.1007/s00792-016-0840-z. Epub 2016 Jun 11. Extremophiles. 2016. PMID: 27290727
-
Deregulation of phenylalanine biosynthesis evolved with the emergence of vascular plants.Plant Physiol. 2022 Jan 20;188(1):134-150. doi: 10.1093/plphys/kiab454. Plant Physiol. 2022. PMID: 34633048 Free PMC article.
-
Uncoupling conformational states from activity in an allosteric enzyme.Nat Commun. 2017 Aug 7;8(1):203. doi: 10.1038/s41467-017-00224-0. Nat Commun. 2017. PMID: 28781362 Free PMC article.
-
Soybean AROGENATE DEHYDRATASES (GmADTs): involvement in the cytosolic isoflavonoid metabolon or trans-organelle continuity?Front Plant Sci. 2024 Jan 23;15:1307489. doi: 10.3389/fpls.2024.1307489. eCollection 2024. Front Plant Sci. 2024. PMID: 38322824 Free PMC article.
References
-
- Aravind L, Koonin EV. Gleaning non-trivial structural, functional and evolutionary information about proteins by iterative database searches. Journal of molecular biology. 1999;287:1023–1040. - PubMed
-
- Bentley R. The shikimate pathway--a metabolic tree with many branches. Critical reviews in biochemistry and molecular biology. 1990;25:307–384. - PubMed
-
- Bode R, Melo C, Birnbaum D. Absolute dependence of phenylalanine and tyrosine biosynthetic enzyme on tryptophan in Candida maltosa. Hoppe-Seyler's Zeitschrift fèur physiologische Chemie. 1984;365:799–803. - PubMed
-
- Brèunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta crystallographica. Section D, Biological crystallography. 1998;54:905–921. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

