Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse
- PMID: 18171685
- PMCID: PMC7116389
- DOI: 10.1242/dev.014357
Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse
Abstract
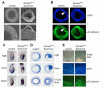
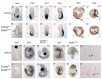
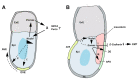
The T-box transcription factor eomesodermin (Eomes) has been implicated as an important component in germ layer induction and patterning in vertebrate embryos. In the mouse, Eomes is essential for development of the trophectoderm lineage and Eomes loss-of-function mutants arrest at implantation. Here, we have used a novel Eomes conditional allele to test Eomes functions in the embryo proper. Eomes-deficient embryos express both Fgf8 and its downstream target Snail at normal levels but surprisingly fail to downregulate E-cadherin. Eomes functional loss thus efficiently and profoundly blocks EMT and concomitant mesoderm delamination. Marker analysis as well as fate-mapping and chimera studies demonstrate for the first time that Eomes is required for specification of the definitive endoderm lineage. We also describe developmental abnormalities in Eomes/Nodal double heterozygotes, and demonstrate that these phenotypes reflect Eomes and Nodal interactions in different tissue sites. Collectively, our experiments establish that Eomes is a key regulator of anteroposterior axis formation, EMT and definitive endoderm specification in the mouse.
Figures





References
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De, Herreros A. The transcription factor snail is a repressor of E- cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. - PubMed
-
- Beck S, Le Good JA, Guzman M, Ben Haim N, Roy K, Beermann F, Constam DB. Extraembryonic proteases regulate Nodal signalling during gastrulation. Nat Cell Biol. 2002;4:981–985. - PubMed
-
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. - PubMed
-
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. - PubMed
-
- Bjornson CR, Griffin KJ, Farr GH, Terashima A, Himeda C, Kikuchi Y, Kimelman D. Eomesodermin is a localized maternal determinant required for endoderm induction in zebrafish. Dev Cell. (3) 2005;9:523–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

