Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis
- PMID: 18171713
- PMCID: PMC3618659
- DOI: 10.2337/db07-0715
Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis
Abstract
Objective: In diabetes, glucose toxicity affects different organ systems, including pancreatic islets where it leads to beta-cell apoptosis, but the mechanisms are not fully understood. Recently, we identified thioredoxin-interacting protein (TXNIP) as a proapoptotic beta-cell factor that is induced by glucose, raising the possibility that TXNIP may play a role in beta-cell glucose toxicity.
Research design and methods: To assess the effects of glucose on TXNIP expression and apoptosis and define the role of TXNIP, we used INS-1 beta-cells; primary mouse islets; obese, diabetic BTBR.ob mice; and a unique mouse model of TXNIP deficiency (HcB-19) that harbors a natural nonsense mutation in the TXNIP gene.
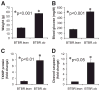
Results: Incubation of INS-1 cells at 25 mmol/l glucose for 24 h led to an 18-fold increase in TXNIP protein, as assessed by immunoblotting. This was accompanied by increased apoptosis, as demonstrated by a 12-fold induction of cleaved caspase-3. Overexpression of TXNIP revealed that TXNIP induces the intrinsic mitochondrial pathway of apoptosis. Islets of diabetic BTBR.ob mice also demonstrated increased TXNIP and apoptosis as did isolated wild-type islets incubated at high glucose. In contrast, TXNIP-deficient HcB-19 islets were protected against glucose-induced apoptosis as measured by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and caspase-3, indicating that TXNIP is a required causal link between glucose toxicity and beta-cell death.
Conclusions: These findings shed new light onto the molecular mechanisms of beta-cell glucose toxicity and apoptosis, demonstrate that TXNIP induction plays a critical role in this vicious cycle, and suggest that inhibition of TXNIP may represent a novel approach to reduce glucotoxic beta-cell loss.
Figures
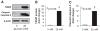

 , 5 mmol/l glucose; ■, 25 mmol/l glucose.
, 5 mmol/l glucose; ■, 25 mmol/l glucose.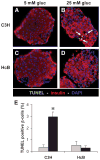
 , 5 mmol/l glucose; ■, 25 mmol/l glucose.
, 5 mmol/l glucose; ■, 25 mmol/l glucose.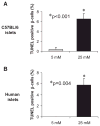

Comment in
-
Thioredoxin-interacting protein is killing my beta-cells!Diabetes. 2008 Apr;57(4):797-8. doi: 10.2337/db08-0055. Diabetes. 2008. PMID: 18375442 No abstract available.
References
-
- Lawrence MC, McGlynn K, Park BH, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J Biol Chem. 2005;280:26751–26759. - PubMed
-
- Khoo S, Gibson TB, Arnette D, Lawrence M, January B, McGlynn K, Vanderbilt CA, Griffen SC, German MS, Cobb MH. MAP Kinases and their roles in pancreatic beta-cells. Cell Biochem Biophys. 2004;40:191–200. - PubMed
-
- Kaiser N, Leibowitz G, Nesher R. Glucotoxicity and beta-cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2003;16:5–22. - PubMed
-
- Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes: a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. - PubMed
-
- Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

