Cerebellar dysfunction explains the extinction-like abolition of conditioned eyeblinks after NBQX injections in the inferior olive
- PMID: 18171918
- PMCID: PMC6671149
- DOI: 10.1523/JNEUROSCI.3403-07.2008
Cerebellar dysfunction explains the extinction-like abolition of conditioned eyeblinks after NBQX injections in the inferior olive
Abstract
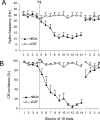
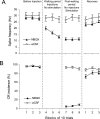
Classical conditioning of the eyeblink response is a form of motor learning that is controlled by the intermediate cerebellum and related brainstem structures. The inferior olive (IO) is commonly thought to provide the cerebellum with a "teaching" unconditioned stimulus (US) signal required for cerebellar learning. Testing this concept has been difficult because the IO, in addition to its putative learning function, also controls tonic activity in the cerebellum. Previously, it was reported that inactivation of AMPA/kainate receptors in the IO produces extinction of conditioned responses (CRs), suggesting that it blocks the transmission of US signals without perturbing the functional state of the cerebellum. However, the electrophysiological support for this critical finding was lacking, mostly because of methodological difficulties in maintaining stable recordings from the same set of single units throughout long drug injection sessions in awake rabbits. To address this critical issue, we used our microwire-based multiple single-unit recording method. The IO in trained rabbits was injected with the AMPA/kainate receptor blocker, 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX), and its effects on CR expression and neuronal activity in the cerebellar interposed nuclei (IN) were examined. We found that NBQX abolished CR expression and that delayed drug effects were independent of the presentation of the conditioned stimulus and were therefore not related to extinction. In parallel to these behavioral effects, the spontaneous neuronal activity and CR-related neuronal responses in the IN were suppressed, suggesting cerebellar dysfunction. These findings indicate that testing the role of IO in learning requires methods that do not alter the functional state of the cerebellum.
Figures





Similar articles
-
Blocking glutamate-mediated inferior olivary signals abolishes expression of conditioned eyeblinks but does not prevent their acquisition.J Neurosci. 2013 May 22;33(21):9097-103. doi: 10.1523/JNEUROSCI.3129-12.2013. J Neurosci. 2013. PMID: 23699520 Free PMC article.
-
Inferior olivary inactivation abolishes conditioned eyeblinks: extinction or cerebellar malfunction?Behav Brain Res. 2007 Mar 12;178(1):128-38. doi: 10.1016/j.bbr.2006.12.012. Epub 2006 Dec 17. Behav Brain Res. 2007. PMID: 17222920
-
Assessing the role of inferior olivary sensory signaling in the expression of conditioned eyeblinks using a combined glutamate/GABAA receptor antagonist protocol.J Neurophysiol. 2012 Jan;107(1):273-82. doi: 10.1152/jn.00705.2011. Epub 2011 Oct 5. J Neurophysiol. 2012. PMID: 21975449 Free PMC article.
-
Associative learning.Int Rev Neurobiol. 1997;41:151-89. doi: 10.1016/s0074-7742(08)60351-7. Int Rev Neurobiol. 1997. PMID: 9378587 Review.
-
Graded error signals in eyeblink conditioning.Neurobiol Learn Mem. 2020 Apr;170:107023. doi: 10.1016/j.nlm.2019.04.011. Epub 2019 Apr 24. Neurobiol Learn Mem. 2020. PMID: 31028891 Review.
Cited by
-
Elimination of the error signal in the superior colliculus impairs saccade motor learning.Proc Natl Acad Sci U S A. 2018 Sep 18;115(38):E8987-E8995. doi: 10.1073/pnas.1806215115. Epub 2018 Sep 5. Proc Natl Acad Sci U S A. 2018. PMID: 30185563 Free PMC article.
-
Quantitative evaluation of posture control in rats with inferior olive lesions.Sci Rep. 2021 Oct 13;11(1):20362. doi: 10.1038/s41598-021-99785-w. Sci Rep. 2021. PMID: 34645901 Free PMC article.
-
Multiple sites of extinction for a single learned response.J Neurophysiol. 2012 Jan;107(1):226-38. doi: 10.1152/jn.00381.2011. Epub 2011 Sep 21. J Neurophysiol. 2012. PMID: 21940608 Free PMC article.
-
Functional relations of cerebellar modules of the cat.J Neurosci. 2010 Jul 14;30(28):9411-23. doi: 10.1523/JNEUROSCI.0440-10.2010. J Neurosci. 2010. PMID: 20631170 Free PMC article.
-
Blocking glutamate-mediated inferior olivary signals abolishes expression of conditioned eyeblinks but does not prevent their acquisition.J Neurosci. 2013 May 22;33(21):9097-103. doi: 10.1523/JNEUROSCI.3129-12.2013. J Neurosci. 2013. PMID: 23699520 Free PMC article.
References
-
- Aksenov D, Serdyukova N, Irwin K, Bracha V. GABA neurotransmission in the cerebellar interposed nuclei: involvement in classically conditioned eyeblinks and neuronal activity. J Neurophysiol. 2004;91:719–727. - PubMed
-
- Batini C, Billard JM, Daniel H. Long-term modification of cerebellar inhibition after inferior olive degeneration. Exp Brain Res. 1985;59:404–409. - PubMed
-
- Bengtsson F, Svensson P, Hesslow G. Feedback control of Purkinje cell activity by the cerebello-olivary pathway. Eur J Neurosci. 2004;20:2999–3005. - PubMed
-
- Bracha V, Webster ML, Winters NK, Irwin KB, Bloedel JR. Effects of muscimol inactivation of the cerebellar interposed-dentate nuclear complex on the performance of the nictitating membrane response. Exp Brain Res. 1994;100:453–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
