Vasopressin increases locomotion through a V1a receptor in orexin/hypocretin neurons: implications for water homeostasis
- PMID: 18171940
- PMCID: PMC6671166
- DOI: 10.1523/JNEUROSCI.3490-07.2008
Vasopressin increases locomotion through a V1a receptor in orexin/hypocretin neurons: implications for water homeostasis
Abstract
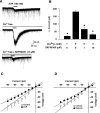
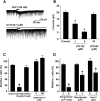
Water homeostasis is a critical challenge to survival for land mammals. Mice display increased locomotor activity when dehydrated, a behavior that improves the likelihood of locating new sources of water and simultaneously places additional demands on compromised hydration levels. The neurophysiology underlying this well known behavior has not been previously elucidated. We report that the anti-diuretic hormone arginine-vasopressin (AVP) is involved in this response. AVP and oxytocin directly induced depolarization and an inward current in orexin/hypocretin neurons. AVP-induced activation of orexin neurons was inhibited by a V1a receptor (V1aR)-selective antagonist and was not observed in V1aR knock-out mice, suggesting an involvement of V1aR. Subsequently activation of phospholipase Cbeta triggers an increase in intracellular calcium by both calcium influx through nonselective cation channels and calcium release from calcium stores in orexin neurons. Intracerebroventricular injection of AVP or water deprivation increased locomotor activity in wild-type mice, but not in transgenic mice lacking orexin neurons. V1aR knock-out mice were less active than wild-type mice. These results suggest that the activation of orexin neurons by AVP or oxytocin has an important role in the regulation of spontaneous locomotor activity in mice. This system appears to play a key role in water deprivation-induced hyperlocomotor activity, a response to dehydration that increases the chance of locating water in nature.
Figures





References
-
- Anthes N, Schmid HA, Hashimoto M, Riediger T, Simon E. Heterogeneous actions of vasopressin on ANG II-sensitive neurons in the subfornical organ of rats. Am J Physiol. 1997;273:R2105–2111. - PubMed
-
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. - PubMed
-
- Arnauld E, Bibene V, Meynard J, Rodriguez F, Vincent JD. Effects of chronic icv infusion of vasopressin on sleep-waking cycle of rats. Am J Physiol. 1989;256:R674–R684. - PubMed
-
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
