Retinocollicular synapse maturation and plasticity are regulated by correlated retinal waves
- PMID: 18171946
- PMCID: PMC6671137
- DOI: 10.1523/JNEUROSCI.4276-07.2008
Retinocollicular synapse maturation and plasticity are regulated by correlated retinal waves
Abstract
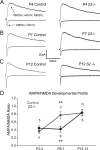
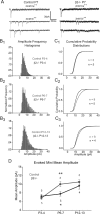
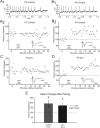
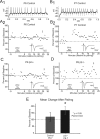
During development, spontaneous retinal waves are thought to provide an instructive signal for retinotopic map formation in the superior colliculus. In mice lacking the beta2 subunit of nicotinic ACh receptors (beta2-/-), correlated retinal waves are absent during the first postnatal week, but return during the second postnatal week. In control retinocollicular synapses, in vitro analysis reveals that AMPA/NMDA ratios and AMPA quantal amplitudes increase during the first postnatal week while the prevalence of silent synapses decreases. In age-matched beta2-/- mice, however, these parameters remain unchanged through the first postnatal week in the absence of retinal waves, but quickly mature to control levels by the end of the second week, suggesting that the delayed onset of correlated waves is able to drive synapse maturation. To examine whether such a mechanistic relationship exists, we applied a "burst-based" plasticity protocol that mimics coincident activity during retinal waves. We find that this pattern of activation is indeed capable of inducing synaptic strengthening [long-term potentiation (LTP)] on average across genotypes early in the first postnatal week [postnatal day 3 (P3) to P4] and, interestingly, that the capacity for LTP at the end of the first week (P6-P7) is significantly greater in immature beta2-/- synapses than in mature control synapses. Together, our results suggest that retinal waves drive retinocollicular synapse maturation through a learning rule that is physiologically relevant to natural wave statistics and that these synaptic changes may serve an instructive role during retinotopic map refinement.
Figures



Similar articles
-
Retinocollicular synaptogenesis and synaptic transmission during formation of the visual map in the superior colliculus of the wallaby (Macropus eugenii).Eur J Neurosci. 2006 Jun;23(11):3043-50. doi: 10.1111/j.1460-9568.2006.04831.x. Eur J Neurosci. 2006. PMID: 16819993
-
Developmental homeostasis of mouse retinocollicular synapses.J Neurosci. 2007 Feb 14;27(7):1746-55. doi: 10.1523/JNEUROSCI.4383-06.2007. J Neurosci. 2007. PMID: 17301182 Free PMC article.
-
Reduced presynaptic efficiency of excitatory synaptic transmission impairs LTP in the visual cortex of BDNF-heterozygous mice.Eur J Neurosci. 2006 Dec;24(12):3519-31. doi: 10.1111/j.1460-9568.2006.05242.x. Eur J Neurosci. 2006. PMID: 17229100
-
Visual experience and maturation of retinal synaptic pathways.Vision Res. 2004 Dec;44(28):3307-16. doi: 10.1016/j.visres.2004.07.041. Vision Res. 2004. PMID: 15535998 Review.
-
Mechanisms of response homeostasis during retinocollicular map formation.J Physiol. 2008 Sep 15;586(18):4363-9. doi: 10.1113/jphysiol.2008.157222. Epub 2008 Jul 10. J Physiol. 2008. PMID: 18617562 Free PMC article. Review.
Cited by
-
Non-cell-autonomous factor induces the transition from excitatory to inhibitory GABA signaling in retina independent of activity.Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22302-7. doi: 10.1073/pnas.1008775108. Epub 2010 Dec 6. Proc Natl Acad Sci U S A. 2010. PMID: 21135238 Free PMC article.
-
Glutamatergic Retinal Waves.Front Neural Circuits. 2016 May 10;10:38. doi: 10.3389/fncir.2016.00038. eCollection 2016. Front Neural Circuits. 2016. PMID: 27242446 Free PMC article. Review.
-
The timing of activity is a regulatory signal during development of neural connections.J Mol Neurosci. 2014 Jul;53(3):324-9. doi: 10.1007/s12031-013-0128-z. Epub 2013 Oct 10. J Mol Neurosci. 2014. PMID: 24114571 Review.
-
Competition driven by retinal waves promotes morphological and functional synaptic development of neurons in the superior colliculus.J Neurophysiol. 2013 Sep;110(6):1441-54. doi: 10.1152/jn.01066.2012. Epub 2013 Jun 5. J Neurophysiol. 2013. PMID: 23741047 Free PMC article.
-
Fine-scale topography in sensory systems: insights from Drosophila and vertebrates.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 Sep;201(9):911-20. doi: 10.1007/s00359-015-1022-7. Epub 2015 Jun 20. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015. PMID: 26091779 Free PMC article. Review.
References
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. - PMC - PubMed
-
- Butts DA. Retinal waves: implications for synaptic learning rules during development. Neuroscientist. 2002;8:243–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
