Heterogeneity in synaptic vesicle release at neuromuscular synapses of mice expressing synaptopHluorin
- PMID: 18171949
- PMCID: PMC6671144
- DOI: 10.1523/JNEUROSCI.3544-07.2008
Heterogeneity in synaptic vesicle release at neuromuscular synapses of mice expressing synaptopHluorin
Abstract
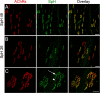
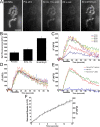
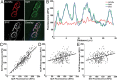
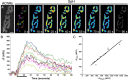
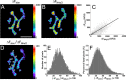
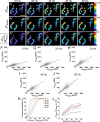
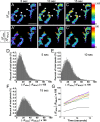
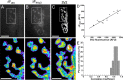
Mammalian neuromuscular junctions are useful model synapses to study the relationship between synaptic structure and function, although these have rarely been studied together at the same synapses. To do this, we generated transgenic lines of mice in which the thy1.2 promoter drives expression of synaptopHluorin (spH) as a means of optically measuring synaptic vesicle distribution and release. SpH is colocalized with other synaptic vesicle proteins in presynaptic terminals and does not alter normal synaptic function. Nerve stimulation leads to readily detectable and reproducible fluorescence changes in motor axon terminals that vary with stimulus frequency and, when compared with electrophysiological recordings, are reliable indicators of neurotransmitter release. Measurements of fluorescence intensity changes reveal a surprising amount of heterogeneity in synaptic vesicle release throughout individual presynaptic motor axon terminals. Some discrete terminal regions consistently displayed a greater rate and extent of release than others, regardless of stimulation frequency. The amount of release at a particular site is highly correlated to the relative abundance of synaptic vesicles there, indicating that a relatively constant fraction of the total vesicular pool, approximately 30%, is released in response to activity. These studies reveal previously unknown relationships between synaptic structure and function at mammalian neuromuscular junctions and demonstrate the usefulness of spH expressing mice as a tool for studying neuromuscular synapses in adults, as well as during development and diseases that affect neuromuscular synaptic function.
Figures
References
-
- Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. - PubMed
-
- Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997;71:3–9. - PubMed
-
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
