Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae
- PMID: 18172022
- PMCID: PMC2262993
- DOI: 10.1091/mbc.e07-08-0827
Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae
Erratum in
- Mol Biol Cell. 2008 May;19(5):2348
Abstract
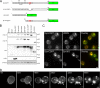
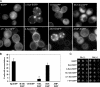
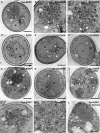
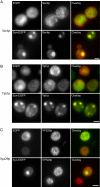
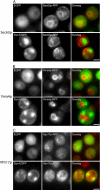
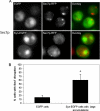
Aggregated alpha-synuclein (alpha-syn) fibrils form Lewy bodies (LBs), the signature lesions of Parkinson's disease (PD) and related synucleinopathies, but the pathogenesis and neurodegenerative effects of LBs remain enigmatic. Recent studies have shown that when overexpressed in Saccharomyces cerevisiae, alpha-syn localizes to plasma membranes and forms cytoplasmic accumulations similar to human alpha-syn inclusions. However, the exact nature, composition, temporal evolution, and underlying mechanisms of yeast alpha-syn accumulations and their relevance to human synucleinopathies are unknown. Here we provide ultrastructural evidence that alpha-syn accumulations are not comprised of LB-like fibrils, but are associated with clusters of vesicles. Live-cell imaging showed alpha-syn initially localized to the plasma membrane and subsequently formed accumulations in association with vesicles. Imaging of truncated and mutant forms of alpha-syn revealed the molecular determinants and vesicular trafficking pathways underlying this pathological process. Because vesicular clustering is also found in LB-containing neurons of PD brains, alpha-syn-mediated vesicular accumulation in yeast represents a model system to study specific aspects of neurodegeneration in PD and related synucleinopathies.
Figures

References
-
- Abeliovich A., et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Chandra S., Gallardo G., Fernandez-Chacon R., Schluter O. M., Sudhof T. C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
-
- Chartier-Harlin M. C., et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. - PubMed
-
- Cole N. B., Murphy D. D., Grider T., Rueter S., Brasaemle D., Nussbaum R. L. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J. Biol. Chem. 2002;277:6344–6352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

