Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions
- PMID: 18172031
- PMCID: PMC2705289
- DOI: 10.1161/CIRCULATIONAHA.107.719765
Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions
Abstract
Background: Monocytes play a key role in atherogenesis, but their participation has been discerned largely via ex vivo analyses of atherosclerotic lesions. We sought to establish a noninvasive technique to determine monocyte trafficking to atherosclerotic lesions in live animals.
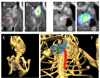
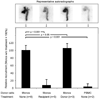
Methods and results: Using a micro-single-photon emission computed tomography small-animal imaging system and a Food and Drug Administration-approved radiotracer ([indium 111] oxyquinoline, (111)In-oxine), we demonstrate here that monocyte recruitment to atherosclerotic lesions can be visualized in a noninvasive, dynamic, and 3-dimensional fashion in live animals. We show in vivo that monocytes are recruited avidly to plaques within days of adoptive transfer. Using micro-single-photon emission computed tomography imaging as a screening tool, we were able to investigate modulatory effects on monocyte recruitment in live animals. We found that 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors rapidly and substantially reduce monocyte recruitment to existing atherosclerotic lesions, as imaged here in vivo.
Conclusions: This novel approach to track monocytes to atherosclerotic plaques in vivo should have broad applications and create new insights into the pathogenesis of atherosclerosis and other inflammatory diseases.
Conflict of interest statement
None.
Figures

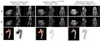

References
-
- Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. - PubMed
-
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. - PubMed
-
- Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. - PubMed
-
- Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;116(Suppl 6A):9S–16S. - PubMed
-
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

