Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease
- PMID: 18172072
- PMCID: PMC2574421
- DOI: 10.1167/iovs.07-0685
Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease
Abstract
Purpose: Squamous metaplasia occurs in ocular surface diseases like Sjögren's syndrome (SS). It is a phenotypic change whereby epithelial cells initiate synthesis of squamous cell-specific proteins such as small proline-rich protein 1B (SPRR1B) that result in pathologic keratin formation on the ocular surface. The authors hypothesized that inflammation is a key inducer of pathologic keratinization and that SPRR1B represents an analytical biomarker for the study of the molecular mechanisms.
Methods: Real-time quantitative RT-PCR and immunohistochemistry were used to examine SPRR1B mRNA and protein in two different mouse models of dry eye and patients with SS. Adoptive transfer of mature lymphocytes from mice lacking the autoimmune regulator (aire) gene was performed to examine the role of inflammation as an inducer of squamous metaplasia. SPRR1B expression in response to several cytokines was examined in vitro, whereas the expression of cytokines IL1beta and IFNgamma was quantified in ocular tissues of aire-deficient mice and patients with SS.
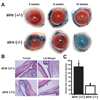
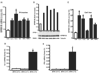
Results: SPRR1B was increased across the ocular surface of mice with both desiccating stress and autoimmune-mediated, aqueous-deficient dry eye and in patients with SS. Adoptive transfer of CD4(+) T cells from aire-deficient mice to immunodeficient recipients caused advanced ocular surface keratinization. IL1alpha, IL1beta, IL6, IFNgamma, and TNFalpha induced SPRR1B expression in vitro and the local expression of IL1beta and IFNgamma was elevated in ocular tissues of patients with SS and aire-deficient mice.
Conclusions: SPRR1B is a valid biomarker for the study of the molecular mechanisms of squamous metaplasia. There is a definitive link between inflammation and squamous metaplasia in autoimmune-mediated dry eye disease, with IL1beta and IFNgamma likely acting as key participants.
Figures





References
-
- Nakamura T, Nishida K, Dota A, Kinoshita S. Changes in conjunctival clusterin expression in severe ocular surface disease. Invest Ophthalmol Vis Sci. 2002;43:1702–1707. - PubMed
-
- Tsubota K, Satake Y, Kaido M, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697–1703. - PubMed
-
- Sacks EH, Jakobiec FA, Wieczorek R, Donnenfeld E, Perry H, Knowles DM., Jr Immunophenotypic analysis of the inflammatory infiltrate in ocular cicatricial pemphigoid: further evidence for a T cell-mediated disease. Ophthalmology. 1989;96:236–243. - PubMed
-
- Raphael M, Bellefqih S, Piette JC, Le Hoang P, Debre P, Chomette G. Conjunctival biopsy in Sjögren’s syndrome: correlations between histological and immunohistochemical features. Histopathology. 1988;13:191–202. - PubMed
-
- Pflugfelder SC, Huang AJ, Feuer W, Chuchovski PT, Pereira IC, Tseng SC. Conjunctival cytologic features of primary Sjögren’s syndrome. Ophthalmology. 1990;97:985–991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

