Effect of eye pigmentation on transscleral drug delivery
- PMID: 18172110
- PMCID: PMC3324932
- DOI: 10.1167/iovs.07-0214
Effect of eye pigmentation on transscleral drug delivery
Abstract
Purpose: To determine the influence of eye pigmentation on transscleral retinal delivery of celecoxib.
Methods: Melanin content in ocular tissues of both the strains was determined by sodium hydroxide solubilization
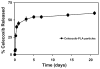
Method: The affinity of celecoxib to synthetic and natural melanin was estimated by co-incubating celecoxib and melanin in isotonic phosphate-buffered saline. The binding affinity (k) and the maximum binding (r(max)) for celecoxib to both natural and synthetic melanin were estimated. Suspension of celecoxib (3 mg/rat) was injected periocularly into one eye of Sprague-Dawley (SD, albino) and Brown Norway (BN, pigmented) rats. The animals were euthanatized at the end of 0.25, 0.5, 1, 2, 3, 4, 8, or 12 hours after the drug was administered, and celecoxib levels in ocular tissues (sclera, choroid-RPE, retina, vitreous, lens, and cornea) were estimated with an HPLC assay. In addition, celecoxib-poly(lactide) microparticles (750 microg drug/rat) were administered periocularly in SD and BN rats, and celecoxib levels in these eye tissues were assessed on day 8, to determine the effectiveness of the sustained release system.
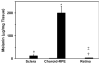
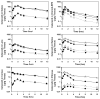
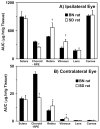
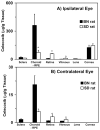
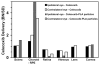
Results: The r(max) and k for celecoxib's binding to natural melanin were (3.92 +/- 0.06) x 10(-7) moles/mg of melanin and (0.08 +/- 0.01) x 10(6) M(-1), respectively. The affinity and the extent of celecoxib's binding to natural melanin were not significantly different from those observed with synthetic melanin. The concentrations of melanin in choroid-RPE, sclera, and retina of BN rats were 200 +/- 30, 12 +/- 4, and 3 +/- 0.2 mug/mg tissue, respectively. Melanin was not detectable in the vitreous, lens, and cornea of BN rats. In SD rats, melanin was not detected in all tissues assessed except in the choroid-RPE, wherein melanin-like activity was 100-fold less than in BN rats. The area under the curve (AUC) for tissue concentration versus time profiles for animals administered with celecoxib suspension was not significantly different between the two strains for sclera, cornea, and lens. However, the retinal (P = 0.001) and vitreal (P = 0.001) AUCs of celecoxib in the treated eyes were approximately 1.5-fold higher in SD rats than in BN rats. Further, the choroid-RPE AUC in the treated and untreated eyes, respectively, were 1.5-fold (P = 0.001) and 2-fold (P = 0.0001) higher in BN rats than in SD rats. With celecoxib-poly(lactide) microparticles, choroid-RPE, retina, and vitreous concentrations on day 8 exhibited similar trends in differences between the two strains, with the differences being greater than those recorded for the celecoxib suspension.
Conclusions: Transscleral retinal and vitreal drug delivery of lipophilic celecoxib is significantly lower in pigmented rats than in albino rats. This difference may be attributable to significant binding of celecoxib to melanin and its accumulation/retention in the melanin-rich choroid-RPE of pigmented rats. The hindrance of retinal and vitreal drug delivery by the choroid-RPE in pigmented rats is also true of sustained-release microparticle systems.
Figures
Similar articles
-
Delivery of celecoxib for treating diseases of the eye: influence of pigment and diabetes.Expert Opin Drug Deliv. 2010 May;7(5):631-45. doi: 10.1517/17425241003663236. Expert Opin Drug Deliv. 2010. PMID: 20205602 Free PMC article. Review.
-
Effect of diabetes on transscleral delivery of celecoxib.Pharm Res. 2009 Feb;26(2):404-14. doi: 10.1007/s11095-008-9757-2. Epub 2008 Nov 6. Pharm Res. 2009. PMID: 18987961
-
Hydrophilic prodrug approach for reduced pigment binding and enhanced transscleral retinal delivery of celecoxib.Mol Pharm. 2012 Mar 5;9(3):605-14. doi: 10.1021/mp2005164. Epub 2012 Feb 8. Mol Pharm. 2012. Retraction in: Mol Pharm. 2015 Jul 6;12(7):2558. doi: 10.1021/acs.molpharmaceut.5b00366. PMID: 22256989 Free PMC article. Retracted.
-
Modeling of corneal and retinal pharmacokinetics after periocular drug administration.Invest Ophthalmol Vis Sci. 2008 Jan;49(1):320-32. doi: 10.1167/iovs.07-0593. Invest Ophthalmol Vis Sci. 2008. PMID: 18172109 Free PMC article.
-
Transscleral drug delivery to the posterior eye: prospects of pharmacokinetic modeling.Adv Drug Deliv Rev. 2006 Nov 15;58(11):1164-81. doi: 10.1016/j.addr.2006.07.025. Epub 2006 Sep 16. Adv Drug Deliv Rev. 2006. PMID: 17069929 Review.
Cited by
-
In vitro transport and partitioning of AL-4940, active metabolite of angiostatic agent anecortave acetate, in ocular tissues of the posterior segment.J Ocul Pharmacol Ther. 2010 Apr;26(2):137-46. doi: 10.1089/jop.2009.0132. J Ocul Pharmacol Ther. 2010. PMID: 20415622 Free PMC article.
-
Engineered peptide-drug conjugate provides sustained protection of retinal ganglion cells with topical administration in rats.J Control Release. 2023 Oct;362:371-380. doi: 10.1016/j.jconrel.2023.08.058. Epub 2023 Sep 4. J Control Release. 2023. PMID: 37657693 Free PMC article.
-
Fluorogenic affinity label for the facile, rapid imaging of proteins in live cells.Org Biomol Chem. 2009 Oct 7;7(19):3969-75. doi: 10.1039/b907664f. Epub 2009 Jul 31. Org Biomol Chem. 2009. PMID: 19763299 Free PMC article.
-
Ocular drug delivery.AAPS J. 2010 Sep;12(3):348-60. doi: 10.1208/s12248-010-9183-3. Epub 2010 May 1. AAPS J. 2010. PMID: 20437123 Free PMC article. Review.
-
Delivery of celecoxib for treating diseases of the eye: influence of pigment and diabetes.Expert Opin Drug Deliv. 2010 May;7(5):631-45. doi: 10.1517/17425241003663236. Expert Opin Drug Deliv. 2010. PMID: 20205602 Free PMC article. Review.
References
-
- Marra M, Gukasyan HJ, Raghava S, Kompella UB. 2nd Ophthalmic Drug Development and Delivery Summit San Diego, CA, 19–20 September 2006. Expert Opin Drug Deliv. 2007;4:77–85. - PubMed
-
- Maurice DM. Drug delivery to the posterior segment from drops. Surv Ophthalmol. 2002;47:S41–S52. - PubMed
-
- Ambati J, Adamis AP. Transscleral drug delivery to the retina and choroid. Prog Retin Eye Res. 2002;21:145–151. - PubMed
-
- Geroski DH, Edelhauser HF. Transscleral drug delivery for posterior segment disease. Adv Drug Deliv Rev. 2001;52:37–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

