DNA protein kinase-dependent G2 checkpoint revealed following knockdown of ataxia-telangiectasia mutated in human mammary epithelial cells
- PMID: 18172300
- PMCID: PMC2664074
- DOI: 10.1158/0008-5472.CAN-07-0675
DNA protein kinase-dependent G2 checkpoint revealed following knockdown of ataxia-telangiectasia mutated in human mammary epithelial cells
Abstract
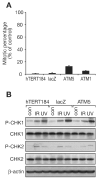
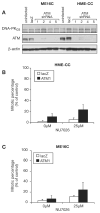
Members of the phosphatidylinositol 3-kinase-related kinase family, in particular the ataxia-telangiectasia mutated (ATM) kinase and the catalytic subunit of the DNA-dependent protein kinase (DNA-PKcs), regulate cellular responses to DNA double-strand breaks. Increased sensitivity to ionizing radiation (IR) in DNA-PKcs- or ATM-deficient cells emphasizes their important roles in maintaining genome stability. Furthermore, combined knockout of both kinases is synthetically lethal, suggesting functional complementarity. In the current study, using human mammary epithelial cells with ATM levels stably knocked down by >90%, we observed an IR-induced G(2) checkpoint that was only slightly attenuated. In marked contrast, this G(2) checkpoint was significantly attenuated with either DNA-PK inhibitor treatment or RNA interference knockdown of DNA-PKcs, the catalytic subunit of DNA-PK, indicating that DNA-PK contributes to the G(2) checkpoint in these cells. Furthermore, in agreement with the checkpoint attenuation, DNA-PK inhibition in ATM-knockdown cells resulted in reduced signaling of the checkpoint kinase CHK1 as evidenced by reduced CHK1 phosphorylation. Taken together, these results show a DNA-PK-dependent component to the IR-induced G(2) checkpoint, in addition to the well-defined ATM-dependent component. This may have important implications for chemotherapeutic strategies for breast cancers.
Figures


References
-
- Niida H, Nakanishi M. DNA damage checkpoints in mammals. Mutagenesis. 2006;21(1):3–9. - PubMed
-
- Meek K, Gupta S, Ramsden DA, Lees-Miller SP. The DNA-dependent protein kinase: the director at the end. Immunol Rev. 2004;200:132–41. - PubMed
-
- Lavin MF, Birrell G, Chen P, Kozlov S, Scott S, Gueven N. ATM signaling and genomic stability in response to DNA damage. Mutat Res. 2005;569(12):123–32. - PubMed
-
- Shinohara ET, Geng L, Tan J, et al. DNA-dependent protein kinase is a molecular target for the development of noncytotoxic radiation-sensitizing drugs. Cancer Res. 2005;65(12):4987–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

