Noninvasive bioluminescence imaging in small animals
- PMID: 18172337
- PMCID: PMC2614121
- DOI: 10.1093/ilar.49.1.103
Noninvasive bioluminescence imaging in small animals
Abstract
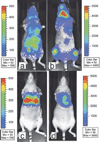
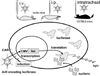
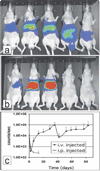
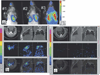
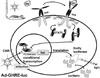
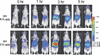
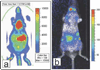
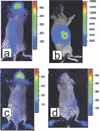
There has been a rapid growth of bioluminescence imaging applications in small animal models in recent years, propelled by the availability of instruments, analysis software, reagents, and creative approaches to apply the technology in molecular imaging. Advantages include the sensitivity of the technique as well as its efficiency, relatively low cost, and versatility. Bioluminescence imaging is accomplished by sensitive detection of light emitted following chemical reaction of the luciferase enzyme with its substrate. Most imaging systems provide 2-dimensional (2D) information in rodents, showing the locations and intensity of light emitted from the animal in pseudo-color scaling. A 3-dimensional (3D) capability for bioluminescence imaging is now available, but is more expensive and less efficient; other disadvantages include the requirement for genetically encoded luciferase, the injection of the substrate to enable light emission, and the dependence of light signal on tissue depth. All of these problems make it unlikely that the method will be extended to human studies. However, in small animal models, bioluminescence imaging is now routinely applied to serially detect the location and burden of xenografted tumors, or identify and measure the number of immune or stem cells after an adoptive transfer. Bioluminescence imaging also makes it possible to track the relative amounts and locations of bacteria, viruses, and other pathogens over time. Specialized applications of bioluminescence also follow tissue-specific luciferase expression in transgenic mice, and monitor biological processes such as signaling or protein interactions in real time. In summary, bioluminescence imaging has become an important component of biomedical research that will continue in the future.
Figures





References
-
- Azadniv M, Dugger K, Bowers WJ, Weaver C, Crispe IN. Imaging CD8+ T cell dynamics in vivo using a transgenic luciferase reporter. Immunol. 2007 (in press). - PubMed
-
- Buchsbaum DJ, Chaudhuri TR, Yamamoto M, Zinn KR. Gene expression imaging with radiolabeled peptides. Ann Nucl Med. 2004;18:275–283. - PubMed
-
- Buchsbaum DJ, Chaudhuri TR, Zinn KR. Radiotargeted gene therapy. J Nucl Med. 2005;46 Suppl 1:179S–186S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

