In vivo evaluation of the HeartWare centrifugal ventricular assist device
- PMID: 18172519
- PMCID: PMC2170489
In vivo evaluation of the HeartWare centrifugal ventricular assist device
Abstract
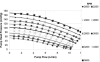
In this study, long-term (90-day) hemocompatibility and end-organ effects of a centrifugal left ventricular assist device (the Heartware HVAD) were evaluated in 6 healthy sheep. The device was implanted into the left ventricular apex on beating hearts. The outflow graft of each device was anastomosed to the descending aorta. None of the sheep received anticoagulation or antiaggregation medication during the study. Hematologic and biochemical tests of liver and kidney function were performed pre-operatively (baseline) and throughout the study. Data associated with pump function were collected continuously until 90 +/- 1 days of support, at which time the sheep were humanely killed, and the end-organs were examined macroscopically and histopathologically. Hematologic and biochemical test results were within normal limits during the study period. There were no significant complications. Postmortem examination of the explanted organs revealed no evidence of ischemia or infarction, except in 2 sheep, in which small foci of infarction were detected in each of their left kidneys. There was no significant device failure. In all sheep, the pump's inflow and outflow conduits were free of thrombus. During the 90-day study, the HeartWare HVAD showed exceptional hemocompatibility and reliability, both of which are crucial to the clinical success of any implantable left ventricular assist device.
Keywords: Animals; assisted circulation/instrumentation; blood pump, centrifugal; equipment design; heart assist device, left ventricular; hemodynamic processes; heparin; sheep; thrombosis/prevention & control.
Figures


References
-
- Dembitsky WP, Tector AJ, Park S, Moskowitz AJ, Gelijns AC, Ronan NS, et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH trial. Ann Thorac Surg 2004;78:2123–30. - PubMed
-
- Digiorgi PL, Reel MS, Thornton B, Burton E, Naka Y, Oz MC. Heart transplant and left ventricular assist device costs. J Heart Lung Transplant 2005;24:200–4. - PubMed
-
- Radovancevic B, Vrtovec B, Frazier OH. Left ventricular assist devices: an alternative to medical therapy for end-stage heart failure. Curr Opin Cardiol 2003;18:210–4. - PubMed
-
- Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med 2001; 345:1435–43. - PubMed
-
- Schmid C, Welp H, Klotz S, Baba HA, Wilhelm MJ, Scheld HH. Outcome of patients surviving to heart transplantation after being mechanically bridged for more than 100 days. J Heart Lung Transplant 2003;22:1054–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
