IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1
- PMID: 18172551
- PMCID: PMC2157566
- DOI: 10.1172/JCI33189
IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1
Abstract
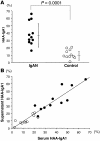
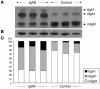
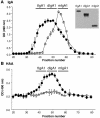
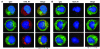
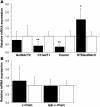
Aberrant glycosylation of IgA1 plays an essential role in the pathogenesis of IgA nephropathy. This abnormality is manifested by a deficiency of galactose in the hinge-region O-linked glycans of IgA1. Biosynthesis of these glycans occurs in a stepwise fashion beginning with the addition of N-acetylgalactosamine by the enzyme N-acetylgalactosaminyltransferase 2 and continuing with the addition of either galactose by beta1,3-galactosyltransferase or a terminal sialic acid by a N-acetylgalactosamine-specific alpha2,6-sialyltransferase. To identify the molecular basis for the aberrant IgA glycosylation, we established EBV-immortalized IgA1-producing cells from peripheral blood cells of patients with IgA nephropathy. The secreted IgA1 was mostly polymeric and had galactose-deficient O-linked glycans, characterized by a terminal or sialylated N-acetylgalactosamine. As controls, we showed that EBV-immortalized cells from patients with lupus nephritis and healthy individuals did not produce IgA with the defective galactosylation pattern. Analysis of the biosynthetic pathways in cloned EBV-immortalized cells from patients with IgA nephropathy indicated a decrease in beta1,3-galactosyltransferase activity and an increase in N-acetylgalactosamine-specific alpha2,6-sialyltransferase activity. Also, expression of beta1,3-galactosyltransferase was significantly lower, and that of N-acetylgalactosamine-specific alpha2,6-sialyltransferase was significantly higher than the expression of these genes in the control cells. Thus, our data suggest that premature sialylation likely contributes to the aberrant IgA1 glycosylation in IgA nephropathy and may represent a new therapeutic target.
Figures




References
-
- Julian B.A., Waldo F.B., Rifai A., Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am. J. Med. 1988;84:129–132. - PubMed
-
- Emancipator, S.N., Mestecky, J., and Lamm, M.E. 2005. IgA nephropathy and related diseases. InMucosal immunology. 3rd edition. J. Mestecky, et al., editors. Elsevier. Amsterdam, The Netherlands. 1579–1600.
-
- Jennette J.C. The immunohistology of IgA nephropathy. Am. J. Kidney Dis. 1988;12:348–352. - PubMed
-
- Allen A.C., et al. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. . Kidney Int. 2001;60:969–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR49084/AR/NIAMS NIH HHS/United States
- DK78244/DK/NIDDK NIH HHS/United States
- DK71802/DK/NIDDK NIH HHS/United States
- M01 RR00211/RR/NCRR NIH HHS/United States
- DK64400/DK/NIDDK NIH HHS/United States
- R24 DK064400/DK/NIDDK NIH HHS/United States
- R01 DK078244/DK/NIDDK NIH HHS/United States
- M01 RR000211/RR/NCRR NIH HHS/United States
- DK61525/DK/NIDDK NIH HHS/United States
- DK47322/DK/NIDDK NIH HHS/United States
- R01 DK071802/DK/NIDDK NIH HHS/United States
- M01 RR000032/RR/NCRR NIH HHS/United States
- R01 DK047322/DK/NIDDK NIH HHS/United States
- P01 DK061525/DK/NIDDK NIH HHS/United States
- M01RR00032/RR/NCRR NIH HHS/United States
- R56 DK078244/DK/NIDDK NIH HHS/United States
- P01 AR049084/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

