Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia
- PMID: 18172553
- PMCID: PMC2157563
- DOI: 10.1172/JCI32702
Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia
Abstract
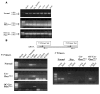
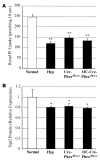
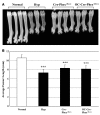
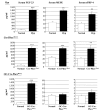
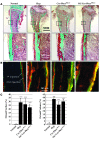
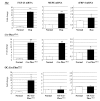
Patients with X-linked hypophosphatemia (XLH) and the hyp-mouse, a model of XLH characterized by a deletion in the Phex gene, manifest hypophosphatemia, renal phosphate wasting, and rickets/osteomalacia. Cloning of the PHEX/Phex gene and mutations in affected patients and hyp-mice established that alterations in PHEX/Phex expression underlie XLH. Although PHEX/Phex expression occurs primarily in osteoblast lineage cells, transgenic Phex expression in hyp-mouse osteoblasts fails to rescue the phenotype, suggesting that Phex expression at other sites underlies XLH. To establish whether abnormal Phex in osteoblasts and/or osteocytes alone generates the HYP phenotype, we created mice with a global Phex knockout (Cre-PhexDeltaflox/y mice) and conditional osteocalcin-promoted (OC-promoted) Phex inactivation in osteoblasts and osteocytes (OC-Cre-PhexDeltaflox/y). Serum phosphorus levels in Cre-PhexDeltaflox/y, OC-Cre-PhexDeltaflox/y, and hyp-mice were lower than those in normal mice. Kidney cell membrane phosphate transport in Cre-PhexDeltaflox/y, OC-Cre-PhexDeltaflox/y, and hyp-mice was likewise reduced compared with that in normal mice. Abnormal renal phosphate transport in Cre-PhexDeltaflox/y and OC-Cre-PhexDeltaflox/y mice was associated with increased bone production and serum FGF-23 levels and decreased kidney membrane type IIa sodium phosphate cotransporter protein, as was the case in hyp-mice. In addition, Cre-PhexDeltaflox/y, OC-Cre-PhexDeltaflox/y, and hyp-mice manifested comparable osteomalacia. These data provide evidence that aberrant Phex function in osteoblasts and/or osteocytes alone is sufficient to underlie the hyp-mouse phenotype.
Figures
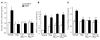


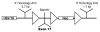
References
-
- Rasmussen, H., and Tenenhouse, H.S. 1995. Mendelian hypophosphatemias. In The metabolic and molecular basis of inherited disease. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill. New York, New York, USA. 3717–3745.
-
- Du L., et al. cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics. 1996;36:22–28. - PubMed
-
- Rowe P.S., et al. Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP). Hum. Mol. Genet. 1997;6:539–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12 RR017614/RR/NCRR NIH HHS/United States
- M01-RR03186-21/RR/NCRR NIH HHS/United States
- P30 AR046031/AR/NIAMS NIH HHS/United States
- P30-AR046031/AR/NIAMS NIH HHS/United States
- R01-AR49410/AR/NIAMS NIH HHS/United States
- M01 RR003186/RR/NCRR NIH HHS/United States
- R21 DK077669/DK/NIDDK NIH HHS/United States
- 5R01-AR051598-05/AR/NIAMS NIH HHS/United States
- 7R03-DE015900-03/DE/NIDCR NIH HHS/United States
- R01 AR051598/AR/NIAMS NIH HHS/United States
- R01-DK65830/DK/NIDDK NIH HHS/United States
- R01-AR27032-26/AR/NIAMS NIH HHS/United States
- R01 DK076829/DK/NIDDK NIH HHS/United States
- R03 DE015900/DE/NIDCR NIH HHS/United States
- R01-SK65830-1/PHS HHS/United States
- 1K12RR017614/RR/NCRR NIH HHS/United States
- R01 AR049410/AR/NIAMS NIH HHS/United States
- R01 AR027032/AR/NIAMS NIH HHS/United States
- R01 DK065830/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

