IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis
- PMID: 18172556
- PMCID: PMC2157567
- DOI: 10.1172/JCI33194
IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis
Abstract
Expression of IL-22 is induced in several human inflammatory conditions, including inflammatory bowel disease (IBD). Expression of the IL-22 receptor is restricted to innate immune cells; however, the role of IL-22 in colitis has not yet been defined. We developed what we believe to be a novel microinjection-based local gene-delivery system that is capable of targeting the inflamed intestine. Using this approach, we demonstrated a therapeutic potency for IL-22-mediated activation of the innate immune pathway in a mouse model of Th2-mediated colitis that induces disease with characteristics similar to that of IBD ulcerative colitis (UC). IL-22 gene delivery enhanced STAT3 activation specifically within colonic epithelial cells and induced both STAT3-dependent expression of mucus-associated molecules and restitution of mucus-producing goblet cells. Importantly, IL-22 gene delivery led to rapid amelioration of local intestinal inflammation. The amelioration of disease by IL-22 was mediated by enhanced mucus production. In addition, local gene delivery was used to inhibit IL-22 activity through overexpression of IL-22-binding protein. Treatment with IL-22-binding protein suppressed goblet cell restitution during the recovery phase of a dextran sulfate sodium-induced model of acute colitis. These data demonstrate what we believe to be a novel function for IL-22 in the intestine and suggest the potency of a local IL-22 gene-delivery system for treating UC.
Figures
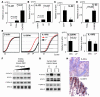
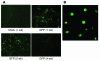

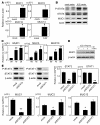

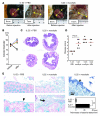
Comment in
-
IL-22: a two-headed cytokine in IBD?Inflamm Bowel Dis. 2009 Mar;15(3):473-4. doi: 10.1002/ibd.20625. Inflamm Bowel Dis. 2009. PMID: 18668680 No abstract available.
References
-
- Dumoutier L., Louahed J., Renauld J.C. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol. 2000;164:1814–1819. - PubMed
-
- Fickenscher H., et al. The interleukin-10 family of cytokines. Trends Immunol. 2002;23:89–96. - PubMed
-
- Pestka S., et al. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 2004;22:929–979. - PubMed
-
- Zheng Y., et al. Interleukin-22, a Th17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

