Effects of disturbed flow on endothelial cells
- PMID: 18172767
- PMCID: PMC3718045
- DOI: 10.1007/s10439-007-9426-3
Effects of disturbed flow on endothelial cells
Erratum in
- Ann Biomed Eng. 2010 Mar;38(3):1258
Abstract
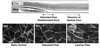
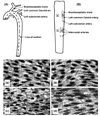
Vascular endothelial cells (ECs) play significant roles in regulating circulatory functions. The shear stress resulting from blood flow modulates EC functions by activating mechano-sensors, signaling pathways, and gene and protein expressions. Shear stress with a clear direction resulting form pulsatile or steady flow causes only transient activation of pro-inflammatory and proliferative pathways, which become down-regulated when such directed shearing is sustained. In contrast, shear flow without a definitive direction (e.g., disturbed flow in regions of complex geometry) causes sustained molecular signaling of pro-inflammatory and proliferative pathways. The EC responses to shear flows with a clear direction involve the remodeling of EC structure to maintain vascular homeostasis and are athero-protective. Such regulatory mechanism does not operate effectively when the flow pattern is disturbed. Therefore, the branch points and other regions of the arterial tree with a complex geometry are prone to atherogenesis, whereas the straight part of the arterial tree is generally spared. Understanding of the EC responses to different flow patters helps to elucidate the mechanism of the region-specific localization of atherosclerosis in the arterial system.
Figures





References
-
- Chen YL, Jan KM, Lin HS, Chien S. Ultrastructural studies on macromolecular permeability in relation to endothelial cell turnover. Atherosclerosis. 1995;118:89–104. - PubMed
-
- Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog. Biophys. Mol. Biol. 2003;83:131–151. - PubMed
-
- Chien S. Molecular basis of rheological modulation of endothelial functions: importance of stress direction. Biorheology. 2006;43:95–116. - PubMed
-
- Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1209–H1224. - PubMed
-
- Chien S, Lin SJ, Weinbaum S, Lee MM, Jan KM. The role of arterial endothelial cell mitosis in macromolecular permeability. Adv. Exp. Med. Biol. 1988;242:59–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

