Metal vinylidenes as catalytic species in organic reactions
- PMID: 18172846
- PMCID: PMC2483426
- DOI: 10.1002/asia.200700247
Metal vinylidenes as catalytic species in organic reactions
Abstract
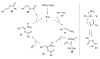
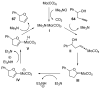
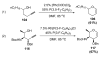
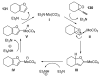
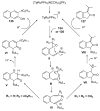
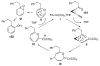
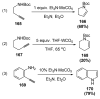
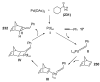
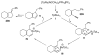
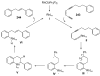
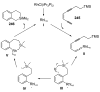
Organic vinylidene species have found limited use in organic synthesis owing to their inaccessibility. In contrast, metal vinylidenes are much more stable and may be readily accessed through transition-metal activation of terminal alkynes. These electrophilic species may be trapped by a number of nucleophiles. Additionally, metal vinylidenes can participate in pericyclic reactions and processes that involve migration of a metal ligand to the vinylidene species. This Focus Review addresses the reactions and applications of metal vinylidenes in organic synthesis.
Figures






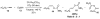


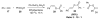

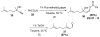

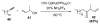



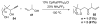




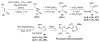








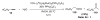










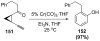

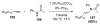

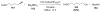


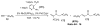

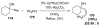




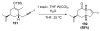
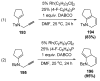

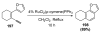
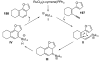

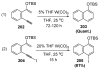
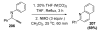
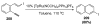




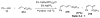


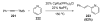

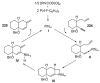
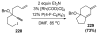
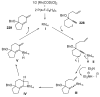
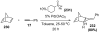




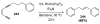
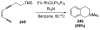
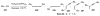







References
-
- Huguet J, Karpf M, Dreiding AS. Tetrahedron Lett. 1983;24:4177–4180.
- Solaja B, Huguet J, Karpf M, Dreiding AS. Tetrahedron. 1987;43:4875–4886.
-
- Bruce MI, Swincer AG. Adv Organomet Chem. 1983;22:59.
- Werner H. Angew Chem Int Ed Engl. 1990;29:1077–1089.
- Bruce MI. Chem Rev. 1991;91:197–257.
- Bruce MI. Chem Rev. 1998;98:2797–2858. - PubMed
-
- Wolf J, Werner H, Serhadli O, Ziegler ML. Angew Chem Int Ed Engl. 1983;22:414–416.
-
-
Unimolecular pathway supported experimentally by double crossover studies: Grotjahn DB, Zeng X, Cooksy AL. J Am Chem Soc. 2006;128:2798–2799.
-
-
-
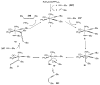
Bimolecular pathway supported by computational studies: Wakatsuki Y, Koga N, Werner H, Morokuma K. J Am Chem Soc. 1997;119:360–366.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

