Cortical dysfunction in patients with Huntington's disease during working memory performance
- PMID: 18172852
- PMCID: PMC6870646
- DOI: 10.1002/hbm.20502
Cortical dysfunction in patients with Huntington's disease during working memory performance
Abstract
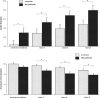
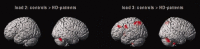
Previous functional neuroimaging studies on executive function suggested multiple functionally aberrant cortical regions in patients with Huntington's disease (HD). However, little is known about the neural mechanisms of working memory (WM) function in this patient population. The objective of this study was to investigate the functional neuroanatomy of WM in HD patients. We used event-related functional magnetic resonance imaging and a parametric verbal WM task to investigate cerebral function during WM performance in 16 healthy control subjects and 12 mild to moderate stage HD patients. We excluded incorrectly performed trials to control for potential accuracy-related activation confounds. Voxel-based morphometry (VBM) was used to control for confounding cortical and subcortical atrophy. We found that HD patients were slower and less accurate than healthy controls across all WM load levels. In addition, HD patients showed lower activation in the left dorso- and ventrolateral prefrontal cortex, the left inferior parietal cortex, the left putamen, and the right cerebellum at high WM load levels only. VBM revealed gray matter differences in the bilateral caudate nucleus and the thalamus, as well as in inferior parietal and right lateral prefrontal regions. However, volumetric abnormalities in the patient group did not affect the activation differences obtained during WM task performance. These findings demonstrate that WM-related functional abnormalities in HD patients involve distinct WM network nodes associated with cognitive control and subvocal rehearsal. Moreover, aberrant cortical function in HD patients may occur in brain regions, which are relatively well preserved in terms of brain atrophy.
(c) 2008 Wiley-Liss, Inc.
Figures


Similar articles
-
Aberrant connectivity of lateral prefrontal networks in presymptomatic Huntington's disease.Exp Neurol. 2008 Sep;213(1):137-44. doi: 10.1016/j.expneurol.2008.05.017. Epub 2008 Jul 11. Exp Neurol. 2008. PMID: 18588876
-
Executive dysfunction in early stages of Huntington's disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study.J Neurol Sci. 2005 Dec 15;239(1):11-9. doi: 10.1016/j.jns.2005.07.007. Epub 2005 Sep 26. J Neurol Sci. 2005. PMID: 16185716
-
Dorsolateral prefrontal cortex dysfunction in presymptomatic Huntington's disease: evidence from event-related fMRI.Brain. 2007 Nov;130(Pt 11):2845-57. doi: 10.1093/brain/awm210. Epub 2007 Sep 13. Brain. 2007. PMID: 17855375
-
[Structural and functional neuroanatomy of attention-deficit hyperactivity disorder (ADHD)].Encephale. 2009 Apr;35(2):107-14. doi: 10.1016/j.encep.2008.01.005. Epub 2008 Jul 7. Encephale. 2009. PMID: 19393378 Review. French.
-
[Functional imaging of cognitive processes in Huntington's disease and its presymptomatic mutation carriers].Nervenarzt. 2008 Apr;79(4):408-20. doi: 10.1007/s00115-007-2390-1. Nervenarzt. 2008. PMID: 18074113 Review. German.
Cited by
-
Advances in Huntington's Disease Biomarkers: A 10-Year Bibliometric Analysis and a Comprehensive Review.Biology (Basel). 2025 Jan 26;14(2):129. doi: 10.3390/biology14020129. Biology (Basel). 2025. PMID: 40001897 Free PMC article. Review.
-
"Frontal" behaviors before the diagnosis of Huntington's disease and their relationship to markers of disease progression: evidence of early lack of awareness.J Neuropsychiatry Clin Neurosci. 2010 Spring;22(2):196-207. doi: 10.1176/jnp.2010.22.2.196. J Neuropsychiatry Clin Neurosci. 2010. PMID: 20463114 Free PMC article.
-
Reduced functional brain connectivity prior to and after disease onset in Huntington's disease.Neuroimage Clin. 2013 Mar 14;2:377-84. doi: 10.1016/j.nicl.2013.03.001. eCollection 2013. Neuroimage Clin. 2013. PMID: 24179791 Free PMC article.
-
Neuroimaging to Facilitate Clinical Trials in Huntington's Disease: Current Opinion from the EHDN Imaging Working Group.J Huntingtons Dis. 2024;13(2):163-199. doi: 10.3233/JHD-240016. J Huntingtons Dis. 2024. PMID: 38788082 Free PMC article. Review.
-
Neural correlates of impaired emotion processing in manifest Huntington's disease.Soc Cogn Affect Neurosci. 2014 May;9(5):671-80. doi: 10.1093/scan/nst029. Epub 2013 Mar 11. Soc Cogn Affect Neurosci. 2014. PMID: 23482620 Free PMC article.
References
-
- Alexander G,Delong M,Strick P ( 1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. - PubMed
-
- Altgassen M,Phillips L,Kopp U,Kliegel M ( 2007): Role of working memory components in planning performance of individuals with Parkinson's disease. Neuropsychologia 45: 2393–2397. - PubMed
-
- Ashburner J,Friston KJ ( 2000): Voxel‐based morphometry—The methods. NeuroImage 11: 805–821. - PubMed
-
- Aylward EH,Li Q,Stine OC,Ranen N,Sherr M,Barta PE,Bylsma FW,Pearlson GD,Ross CA ( 1997): Longitudinal change in basal ganglia volume in patients with Huntington's disease. Neurology 48: 394–399. - PubMed
-
- Baddeley A ( 2000): The episodic buffer: A new component of working memory? Trends Cogn Sci 4: 417–423. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

