Metal-catalyzed 1,2-shift of diverse migrating groups in allenyl systems as a new paradigm toward densely functionalized heterocycles
- PMID: 18173272
- PMCID: PMC3686647
- DOI: 10.1021/ja0773507
Metal-catalyzed 1,2-shift of diverse migrating groups in allenyl systems as a new paradigm toward densely functionalized heterocycles
Abstract
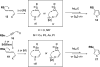
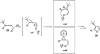
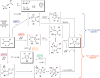
A general, mild, and efficient 1,2-migration/cycloisomerization methodology toward multisubstituted 3-thio-, seleno-, halo-, aryl-, and alkyl-furans and pyrroles, as well as fused heterocycles, valuable building blocks for synthetic chemistry, has been developed. Moreover, regiodivergent conditions have been identified for C-4 bromo- and thio-substituted allenones and alkynones for the assembly of regioisomeric 2-hetero substituted furans selectively. It was demonstrated that, depending on reaction conditions, ambident substrates can be selectively transformed into furan products, as well as undergo selective 6-exo-dig or Nazarov cyclizations. Our mechanistic investigations have revealed that the transformation proceeds via allenylcarbonyl or allenylimine intermediates followed by 1,2-group migration to the allenyl sp carbon during cycloisomerization. It was found that 1,2-migration of chalcogens and halogens predominantly proceeds via formation of irenium intermediates. Analogous intermediate can also be proposed for 1,2-aryl shift. Furthermore, it was shown that the cycloisomerization cascade can be catalyzed by Brønsted acids, albeit less efficiently, and commonly observed reactivity of Lewis acid catalysts cannot be attributed to the eventual formation of proton. Undoubtedly, thermally induced or Lewis acid-catalyzed transformations proceed via intramolecular Michael addition or activation of the enone moiety pathways, whereas certain carbophilic metals trigger carbenoid/oxonium type pathway. However, a facile cycloisomerization in the presence of cationic complexes, as well as observed migratory aptitude in the cycloisomerization of unsymmetrically disubstituted aryl- and alkylallenes, strongly supports electrophilic nature for this transformation. Full mechanistic details, as well as the scope of this transformation, are discussed.
Figures


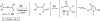
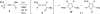
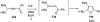
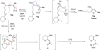

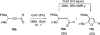
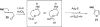

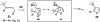
References
-
-
See for example:
- Bellina F, Rossi R. Tetrahedron. 2006;62:7213.
- Hou XL, Yang Z, Wong HNC. In: Progress in Heterocyclic Chemistry. Gribble GW, Gilcrist TL, editors. Vol. 15. Pergamon; Oxford: 2003. p. 167.
- Keay BA, Dibble PW. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Scriven EFV, editors. Vol. 2. Elsevier; Oxford: 1997. p. 395.
- Donnelly DMX, Meegan MJ. In: Comprehensive Heterocyclic Chemistry. Katritzky AR, Rees CW, editors. Vol. 4. Pergamon; Oxford: 1984. p. 657.
- Dean FM. Naturally Occurring Oxygen Ring Compounds. Butterworths; London: 1963. p. 1. Chapter 1.
-
-
-
For most important examples, see:
- Fürstner A, Weintritt H. J Am Chem Soc. 1998;120:2817.
- Fürstner A. Angew Chem., Int Ed. 2003;42:3582. - PubMed
- Fürstner A, Reinecke K, Prinz H, Waldmann H. ChemBioChem. 2004;5:1575. - PubMed
- Fürstner A, Grabowski EJ. Chem Bio Chem. 2001;2:706. - PubMed
- Pettit GR, McNulty J, Herald DL, Doubek DL, Chapuis JC, Schmidt JM, Tackett LP, Boyd MR. J Nat Prod. 1997;60:180. - PubMed
-
-
- Miyata Y, Nishinaga T, Komatsu K. J Org Chem. 2005;70:1147. - PubMed
- Heylen M, Van den Broeck K, Boutton C, van Beylen M, Persoons A, Samyn C. Eur Polym J. 1998;34:1453.
- Ferrero F, Napoli L, Tonin C, Varesano A. J Appl Polym Sci. 2006;102:4121.
- Venkatatraman S, Kumar R, Sankar J, Chandrashekar TK, Senhil K, Vijayan C, Kelling A, Senge MO. Chem.–Eur J. 2004;10:1423. - PubMed
- Facchetti A, Abbotto A, Beverina L, van der Boom ME, Dutta P, Evmenenko G, Pagani GA, Marks TJ. Chem Mater. 2003;15:1064.
- Zhang LZ, Chen CW, Lee CF, Wu CC, Luh TY. Chem Commun. 2002:2336. - PubMed
- Novak P, Müller K, Santhanum KSV, Haas O. Chem Rev. 1997;97:207. - PubMed
-
- Lee HK, Chan KF, Hui CW, Yim HK, Wu XW, Wong HNC. Pure Appl Chem. 2005;77:139.
- Wong HNC, Yu P, Yick CY. Pure Appl Chem. 1999;71:1041.
- Lipshutz BH. Chem Rev. 1986;86:795.
-
-
For recent reviews, see:
- König B. In: Product Class 9: Furans, in Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. Maas G, editor. Georg Thieme; Stuttgart: 2001. pp. 183–285.
- Patil NT, Yamamoto Y. ARKIVOC. 2007;10:121.
- Kirsch SF. Org Biomol Chem. 2006;4:2076. - PubMed
- Brown RCD. Angew Chem., Int Ed. 2005;44:850. - PubMed
- Jeevanandam A, Ghule A, Ling YC. Curr Org Chem. 2002;6:841.
- Keay BA. Chem Soc Rev. 1999;28:209.
- Hou XL, Cheung HY, Hon TY, Kwan PL, Lo TH, Tong SY, Wong HNC. Tetrahedron. 1998;54:1955.
- Schröter S, Stock C, Bach T. Tetrahedron. 2005;61:2245.
- Schmuck C, Rupprecht D. Synthesis. 2007:3095.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

