Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs
- PMID: 18173372
- PMCID: PMC2732400
- DOI: 10.1146/annurev.immunol.24.021605.090620
Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs
Abstract
The immune system is the most diffuse cellular system in the body. Accordingly, long-range migration of cells and short-range communication by local chemical signaling and by cell-cell contacts are vital to the control of an immune response. Cellular homing and migration within lymphoid organs, antigen recognition, and cell signaling and activation are clearly vital during an immune response, but these events had not been directly observed in vivo until recently. Introduced to the field of immunology in 2002, two-photon microscopy is the method of choice for visualizing living cells deep within native tissue environments, and it is now revealing an elegant cellular choreography that underlies the adaptive immune response to antigen challenge. We review cellular dynamics and molecular factors that contribute to basal motility of lymphocytes in the lymph node and cellular interactions leading to antigen capture and recognition, T cell activation, B cell activation, cytolytic effector function, and antibody production.
Conflict of interest statement
Figures

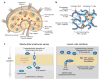
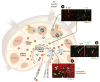
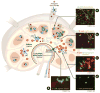
References
-
- Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296:1876–80. - PubMed
-
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–73. - PubMed
-
- Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–76. - PubMed
-
- Petty HR. Fluorescence microscopy: established and emerging methods, experimental strategies, and applications in immunology. Microsc Res Tech. 2007;70:687–709. - PubMed
-
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

