Association of SAP130/SF3b-3 with Cullin-RING ubiquitin ligase complexes and its regulation by the COP9 signalosome
- PMID: 18173839
- PMCID: PMC2265268
- DOI: 10.1186/1471-2091-9-1
Association of SAP130/SF3b-3 with Cullin-RING ubiquitin ligase complexes and its regulation by the COP9 signalosome
Abstract
Background: Cullin-RING ubiquitin E3 ligases (CRLs) are regulated by modification of an ubiquitin-like protein, Nedd8 (also known as Rub1) on the cullin subunit. Neddylation is shown to facilitate E3 complex assembly; while un-neddylated cullins are bound by CAND1 that prevents recruitment of the substrates. The level of Nedd8 modification is critically dependent on the COP9 signalosome (CSN), an eight-subunit protein complex containing Nedd8 isopeptidase activity.
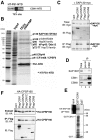
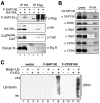
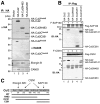
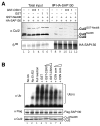
Results: We report isolation of SAP130 (SF3b-3) as a CSN1 interacting protein. SAP130 is homologous to DDB1, and is a component of SF3b RNA splicing complex and STAGA/TFTC transcription complexes, but its specific function within these complexes is unknown. We show that SAP130 can interact with a variety of cullin proteins. It forms tertiary complexes with fully assembled CRL E3 complexes such as SCFSkp2, Elongin B/C -Cul2- VHL and Cul4-DDB complex by binding to both N-terminal and C-terminal domain of cullins. SAP130 preferentially associates with neddylated cullins in vivo. However knock-down of CAND1 abolished this preference and increased association of SAP130 with Cul2. Furthermore, we provide evidence that CSN regulates SAP130-Cul2 interaction and SAP130-associated polyubiquitinating activity.
Conclusion: SAP130 is a cullin binding protein that is likely involved in the Nedd8 pathway. The association of SAP130 with various cullin member proteins such as Cul1, Cul2 and Cul4A is modulated by CAND1 and CSN. As an established component of transcription and RNA processing complexes, we hypothesis that SAP130 may link CRL mediated ubiquitination to gene expression.
Figures

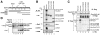
Similar articles
-
CAND1 enhances deneddylation of CUL1 by COP9 signalosome.Biochem Biophys Res Commun. 2005 Sep 2;334(3):867-74. doi: 10.1016/j.bbrc.2005.06.188. Biochem Biophys Res Commun. 2005. PMID: 16036220
-
Deregulation of the COP9 signalosome-cullin-RING ubiquitin-ligase pathway: mechanisms and roles in urological cancers.Int J Biochem Cell Biol. 2013 Jul;45(7):1327-37. doi: 10.1016/j.biocel.2013.03.023. Epub 2013 Apr 10. Int J Biochem Cell Biol. 2013. PMID: 23583660 Review.
-
Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate.Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11515-20. doi: 10.1073/pnas.0603921103. Epub 2006 Jul 21. Proc Natl Acad Sci U S A. 2006. PMID: 16861300 Free PMC article.
-
Protection of cullin-RING E3 ligases by CSN-UBP12.Trends Cell Biol. 2006 Jul;16(7):362-9. doi: 10.1016/j.tcb.2006.05.001. Epub 2006 Jun 9. Trends Cell Biol. 2006. PMID: 16762551 Review.
-
Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation.Nat Cell Biol. 2005 Oct;7(10):1014-20. doi: 10.1038/ncb1301. Epub 2005 Aug 28. Nat Cell Biol. 2005. PMID: 16127432
Cited by
-
Pattern recognition receptors and central nervous system repair.Exp Neurol. 2014 Aug;258:5-16. doi: 10.1016/j.expneurol.2014.01.001. Exp Neurol. 2014. PMID: 25017883 Free PMC article. Review.
-
ABL kinases regulate the stabilization of HIF-1α and MYC through CPSF1.Proc Natl Acad Sci U S A. 2023 Apr 18;120(16):e2210418120. doi: 10.1073/pnas.2210418120. Epub 2023 Apr 11. Proc Natl Acad Sci U S A. 2023. PMID: 37040401 Free PMC article.
-
The SF3b complex: splicing and beyond.Cell Mol Life Sci. 2020 Sep;77(18):3583-3595. doi: 10.1007/s00018-020-03493-z. Epub 2020 Mar 5. Cell Mol Life Sci. 2020. PMID: 32140746 Free PMC article. Review.
-
CSN1 inhibits c-Jun phosphorylation and down-regulates ectopic expression of JNK1.Protein Cell. 2011 May;2(5):423-32. doi: 10.1007/s13238-011-1043-0. Epub 2011 May 21. Protein Cell. 2011. PMID: 21604193 Free PMC article.
-
SAP130 and CSN1 interact and regulate male gametogenesis in Arabidopsis thaliana.J Plant Res. 2021 Mar;134(2):279-289. doi: 10.1007/s10265-021-01260-0. Epub 2021 Feb 8. J Plant Res. 2021. PMID: 33555481
References
-
- Michel J, Xiong Y. Human CUL-1, but not other cullin family members, selectively interacts with SKP1 to form a complex with SKP2 and cyclin A. Cell Growth Differ. 1998;9:435–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

