EmbA is an essential arabinosyltransferase in Mycobacterium tuberculosis
- PMID: 18174142
- PMCID: PMC2885622
- DOI: 10.1099/mic.0.2007/012153-0
EmbA is an essential arabinosyltransferase in Mycobacterium tuberculosis
Abstract
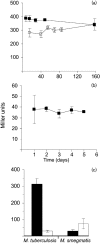
The Emb proteins (EmbA, EmbB, EmbC) are mycobacterial arabinosyltransferases involved in the biogenesis of the mycobacterial cell wall. EmbA and EmbB are predicted to work in unison as a heterodimer. EmbA and EmbB are involved in the formation of the crucial terminal hexaarabinoside motif [Arabeta(1-->2)Araalpha(1-->5)] [Arabeta(1-->2)Araalpha(1-->3)]Araalpha(1-->5)Araalpha1-->(Ara(6)) in the cell wall polysaccharide arabinogalactan. Studies conducted in Mycobacterium smegmatis revealed that mutants with disruptions in embA or embB are viable, although the growth rate was affected. In contrast, we demonstrate here that embA is an essential gene in Mycobacterium tuberculosis, since a deletion of the chromosomal gene could only be achieved when a second functional copy was provided on an integrated vector. Complementation of an embA mutant of M. smegmatis by M. tuberculosis embA confirmed that it encodes a functional arabinosyltransferase. We identified a promoter for M. tuberculosis embA located immediately upstream of the gene, indicating that it is expressed independently from the upstream gene, embC. Promoter activity from P(embA)((Mtb)) was sevenfold lower when assayed in M. smegmatis compared to M. tuberculosis, indicating that the latter is not a good host for genetic analysis of M. tuberculosis embA expression. P(embA)((Mtb)) activity remained constant throughout growth phases and after stress treatment, although it was reduced during hypoxia-induced non-replicating persistence. Ethambutol exposure had no effect on P(embA)((Mtb)) activity. These data demonstrate that M. tuberculosis embA encodes a functional arabinosyltransferase which is constitutively expressed and plays a critical role in M. tuberculosis.
Figures



References
-
- Belanger, A. E., Besra, G. S., Ford, M. E., Mikusova, K., Belisle, J. T., Brennan, P. J. & Inamine, J. M. (1996). The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc Natl Acad Sci U S A 93, 11919–11924. - PMC - PubMed
-
- Berg, S., Torrelles, J. B., Chatterjee, D., Crick, D. C., Escuyer, V. E. & Brennan, P. J. (2003). Point mutations in embC affect synthesis of lipoarabinomannan in Mycobacterium smegmatis. Abstract submitted for the 8th Annual Conference of the Society for Glycobiology. Glycobiology 13, 853
-
- Brennan, P. J. (2003). Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb) 83, 91–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

