A deletion mutant of vitronectin lacking the somatomedin B domain exhibits residual plasminogen activator inhibitor-1-binding activity
- PMID: 18174166
- PMCID: PMC2447658
- DOI: 10.1074/jbc.M708017200
A deletion mutant of vitronectin lacking the somatomedin B domain exhibits residual plasminogen activator inhibitor-1-binding activity
Abstract
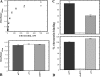
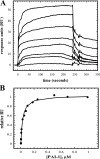
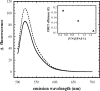
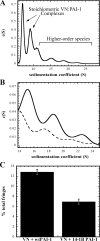
Vitronectin and plasminogen activator inhibitor-1 (PAI-1) are important physiological binding partners that work in concert to regulate cellular adhesion, migration, and fibrinolysis. The high affinity binding site for PAI-1 is located within the N-terminal somatomedin B domain of vitronectin; however, several studies have suggested a second PAI-1-binding site within vitronectin. To investigate this secondary site, a vitronectin mutant lacking the somatomedin B domain (rDeltasBVN) was engineered. The short deletion had no effect on heparin-binding, integrin-binding, or cellular adhesion. Binding to the urokinase receptor was completely abolished while PAI-1 binding was still observed, albeit with a lower affinity. Analytical ultracentrifugation on the PAI-1-vitronectin complex demonstrated that increasing NaCl concentration favors 1:1 versus 2:1 PAI-1-vitronectin complexes and hampers formation of higher order complexes, pointing to the contribution of charge-charge interactions for PAI-1 binding to the second site. Furthermore, fluorescence resonance energy transfer between differentially labeled PAI-1 molecules confirmed that two independent molecules of PAI-1 are capable of binding to vitronectin. These results support a model for the assembly of higher order PAI-1-vitronectin complexes via two distinct binding sites in both proteins.
Figures



References
-
- Preissner, K. T., and Muller-Berghaus, G. (1986) Eur. J. Biochem. 156 645–650 - PubMed
-
- Podor, T. J., Campbell, S., Chindemi, P., Foulon, D. M., Farrell, D. H., Walton, P. D., Weitz, J. I., and Peterson, C. B. (2002) J. Biol. Chem. 277 7520–7528 - PubMed
-
- Xu, D., Baburaj, K., Peterson, C. B., and Xu, Y. (2001) Proteins 44 312–320 - PubMed
-
- Mayasundari, A., Whittemore, N. A., Serpersu, E. H., and Peterson, C. B. (2004) J. Biol. Chem. 279 29359–29366 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

