Species differences in Cl- affinity and in electrogenicity of SLC26A6-mediated oxalate/Cl- exchange correlate with the distinct human and mouse susceptibilities to nephrolithiasis
- PMID: 18174209
- PMCID: PMC2375677
- DOI: 10.1113/jphysiol.2007.143222
Species differences in Cl- affinity and in electrogenicity of SLC26A6-mediated oxalate/Cl- exchange correlate with the distinct human and mouse susceptibilities to nephrolithiasis
Abstract
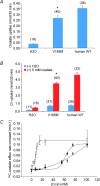
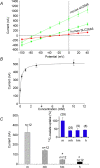
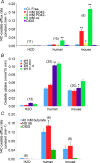
The mouse is refractory to lithogenic agents active in rats and humans, and so has been traditionally considered a poor experimental model for nephrolithiasis. However, recent studies have identified slc26a6 as an oxalate nephrolithiasis gene in the mouse. Here we extend our earlier demonstration of different anion selectivities of the orthologous mouse and human SLC26A6 polypeptides to investigate the correlation between species-specific differences in SLC26A6 oxalate/anion exchange properties as expressed in Xenopus oocytes and in reported nephrolithiasis susceptibility. We find that human SLC26A6 mediates minimal rates of Cl(-) exchange for Cl(-), sulphate or formate, but rates of oxalate/Cl(-) exchange roughly equivalent to those of mouse slc2a6. Both transporters exhibit highly cooperative dependence of oxalate efflux rate on extracellular [Cl(-)], but whereas the K(1/2) for extracellular [Cl(-)] is only 8 mM for mouse slc26a6, that for human SLC26A6 is 62 mM. This latter value approximates the reported mean luminal [Cl(-)] of postprandial human jejunal chyme, and reflects contributions from both transmembrane and C-terminal cytoplasmic domains of human SLC26A6. Human SLC26A6 variant V185M exhibits altered [Cl(-)] dependence and reduced rates of oxalate/Cl(-) exchange. Whereas mouse slc26a6 mediates bidirectional electrogenic oxalate/Cl(-) exchange, human SLC26A6-mediated oxalate transport appears to be electroneutral. We hypothesize that the low extracellular Cl(-) affinity and apparent electroneutrality of oxalate efflux characterizing human SLC26A6 may partially explain the high human susceptibility to nephrolithiasis relative to that of mouse. SLC26A6 sequence variant(s) are candidate risk modifiers for nephrolithiasis.
Figures





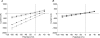
Comment in
-
The role of SLC26A6-mediated chloride/oxalate exchange in causing susceptibility to nephrolithiasis.J Physiol. 2008 Mar 1;586(5):1205-6. doi: 10.1113/jphysiol.2007.150565. J Physiol. 2008. PMID: 18310129 Free PMC article. No abstract available.
Similar articles
-
Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity.J Biol Chem. 2005 Mar 4;280(9):8564-80. doi: 10.1074/jbc.M411703200. Epub 2004 Nov 17. J Biol Chem. 2005. PMID: 15548529
-
The role of SLC26A6-mediated chloride/oxalate exchange in causing susceptibility to nephrolithiasis.J Physiol. 2008 Mar 1;586(5):1205-6. doi: 10.1113/jphysiol.2007.150565. J Physiol. 2008. PMID: 18310129 Free PMC article. No abstract available.
-
Anion exchangers in flux: functional differences between human and mouse SLC26A6 polypeptides.Novartis Found Symp. 2006;273:107-19; discussion 119-25, 261-4. Novartis Found Symp. 2006. PMID: 17120764
-
Essential roles of CFEX-mediated Cl(-)-oxalate exchange in proximal tubule NaCl transport and prevention of urolithiasis.Kidney Int. 2006 Oct;70(7):1207-13. doi: 10.1038/sj.ki.5001741. Epub 2006 Aug 2. Kidney Int. 2006. PMID: 16883319 Review.
-
Role of SLC26A6-mediated Cl⁻-oxalate exchange in renal physiology and pathophysiology.J Nephrol. 2010 Nov-Dec;23 Suppl 16:S158-64. J Nephrol. 2010. PMID: 21170874 Review.
Cited by
-
SLC26 Anion Transporters.Handb Exp Pharmacol. 2024;283:319-360. doi: 10.1007/164_2023_698. Handb Exp Pharmacol. 2024. PMID: 37947907 Review.
-
Functional coupling of apical Cl-/HCO3- exchange with CFTR in stimulated HCO3- secretion by guinea pig interlobular pancreatic duct.Am J Physiol Gastrointest Liver Physiol. 2009 Jun;296(6):G1307-17. doi: 10.1152/ajpgi.90697.2008. Epub 2009 Apr 2. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19342507 Free PMC article.
-
SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization.Am J Physiol Cell Physiol. 2011 Aug;301(2):C289-303. doi: 10.1152/ajpcell.00089.2011. Epub 2011 May 18. Am J Physiol Cell Physiol. 2011. PMID: 21593449 Free PMC article.
-
Nephropathy in dietary hyperoxaluria: A potentially preventable acute or chronic kidney disease.World J Nephrol. 2014 Nov 6;3(4):122-42. doi: 10.5527/wjn.v3.i4.122. World J Nephrol. 2014. PMID: 25374807 Free PMC article. Review.
-
The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man.Urolithiasis. 2017 Feb;45(1):89-108. doi: 10.1007/s00240-016-0952-z. Epub 2016 Dec 2. Urolithiasis. 2017. PMID: 27913853 Free PMC article. Review.
References
-
- Cao LC, Honeyman TW, Cooney R, Kennington L, Scheid CR, Jonassen JA. Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int. 2004;66:1890–1900. - PubMed
-
- Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem. 2005;280:8564–8580. - PubMed
-
- Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Digestive Dis. 1966;11:503–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

