Dendritic glutamate release produces autocrine activation of mGluR1 in cerebellar Purkinje cells
- PMID: 18174329
- PMCID: PMC2206607
- DOI: 10.1073/pnas.0709407105
Dendritic glutamate release produces autocrine activation of mGluR1 in cerebellar Purkinje cells
Abstract
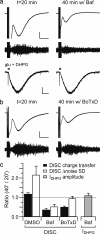
In recent years, it has become clear that, in addition to conventional anterograde transmission, signaling in neural circuits can occur in a retrograde manner. This suggests the additional possibility that postsynaptic release of neurotransmitter might be able to act in an autocrine fashion. Here, we show that brief depolarization of a cerebellar Purkinje cell triggers a slow inward current. This depolarization-induced slow current (DISC) is attenuated by antagonists of mGluR1 or TRP channels. DISC is eliminated by a mixture of voltage-sensitive Ca2+ channel blockers and is mimicked by a brief climbing fiber burst. DISC is attenuated by an inhibitor of vesicular glutamate transporters or of vesicular fusion. These data suggest that Ca2+-dependent postsynaptic fusion of glutamate-loaded vesicles evokes a slow inward current produced by activation of postsynaptic mGluR1, thereby constituting a useful form of feedback regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Somatodendritic release of glutamate regulates synaptic inhibition in cerebellar Purkinje cells via autocrine mGluR1 activation.J Neurosci. 2007 Nov 14;27(46):12464-74. doi: 10.1523/JNEUROSCI.0178-07.2007. J Neurosci. 2007. PMID: 18003824 Free PMC article.
-
Depolarization-induced slow current in cerebellar Purkinje cells does not require metabotropic glutamate receptor 1.Neuroscience. 2009 Sep 1;162(3):688-93. doi: 10.1016/j.neuroscience.2009.01.044. Epub 2009 Jan 29. Neuroscience. 2009. PMID: 19409231 Free PMC article.
-
Ca2+ ion permeability and single-channel properties of the metabotropic slow EPSC of rat Purkinje neurons.J Neurosci. 2004 Apr 7;24(14):3563-73. doi: 10.1523/JNEUROSCI.5374-03.2004. J Neurosci. 2004. PMID: 15071104 Free PMC article.
-
Metabotropic glutamate receptors in the cerebellum with a focus on their function in Purkinje cells.Cerebellum. 2002 Jan-Mar;1(1):19-26. doi: 10.1007/BF02941886. Cerebellum. 2002. PMID: 12879970 Review.
-
Dendritic calcium signaling in cerebellar Purkinje cell.Neural Netw. 2013 Nov;47:11-7. doi: 10.1016/j.neunet.2012.08.001. Epub 2012 Sep 5. Neural Netw. 2013. PMID: 22985934 Review.
Cited by
-
Characterizing the conductance underlying depolarization-induced slow current in cerebellar Purkinje cells.J Neurophysiol. 2013 Feb;109(4):1174-81. doi: 10.1152/jn.01168.2011. Epub 2012 Nov 28. J Neurophysiol. 2013. PMID: 23197456 Free PMC article.
-
Motoneurons regulate the central pattern generator during drug-induced locomotor-like activity in the neonatal mouse.Elife. 2017 Jul 3;6:e26622. doi: 10.7554/eLife.26622. Elife. 2017. PMID: 28671548 Free PMC article.
-
Artifact versus reality--how astrocytes contribute to synaptic events.Glia. 2012 Jul;60(7):1013-23. doi: 10.1002/glia.22288. Epub 2012 Jan 6. Glia. 2012. PMID: 22228580 Free PMC article. Review.
-
Backpropagating action potentials enable detection of extrasynaptic glutamate by NMDA receptors.Cell Rep. 2012 May 31;1(5):495-505. doi: 10.1016/j.celrep.2012.03.007. Epub 2012 Apr 26. Cell Rep. 2012. PMID: 22832274 Free PMC article.
-
Activity-dependent regulation of synapses by retrograde messengers.Neuron. 2009 Jul 30;63(2):154-70. doi: 10.1016/j.neuron.2009.06.021. Neuron. 2009. PMID: 19640475 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

