Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity
- PMID: 18174371
- PMCID: PMC2170513
- DOI: 10.1101/lm.661908
Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity
Abstract
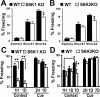
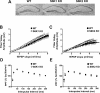
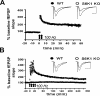
Protein synthesis is required for the expression of enduring memories and long-lasting synaptic plasticity. During cellular proliferation and growth, S6 kinases (S6Ks) are activated and coordinate the synthesis of de novo proteins. We hypothesized that protein synthesis mediated by S6Ks is critical for the manifestation of learning, memory, and synaptic plasticity. We have tested this hypothesis with genetically engineered mice deficient for either S6K1 or S6K2. We have found that S6K1-deficient mice express an early-onset contextual fear memory deficit within one hour of training, a deficit in conditioned taste aversion (CTA), impaired Morris water maze acquisition, and hypoactive exploratory behavior. In contrast, S6K2-deficient mice exhibit decreased contextual fear memory seven days after training, a reduction in latent inhibition of CTA, and normal spatial learning in the Morris water maze. Surprisingly, neither S6K1- nor S6K2-deficient mice exhibited alterations in protein synthesis-dependent late-phase long-term potentiation (L-LTP). However, removal of S6K1, but not S6K2, compromised early-phase LTP expression. Furthermore, we observed that S6K1-deficient mice have elevated basal levels of Akt phosphorylation, which is further elevated following induction of L-LTP. Taken together, our findings demonstrate that removal of S6K1 leads to a distinct array of behavioral and synaptic plasticity phenotypes that are not mirrored by the removal of S6K2. Our observations suggest that neither gene by itself is required for L-LTP but instead may be required for other types of synaptic plasticity required for cognitive processing.
Figures


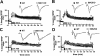

References
-
- Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Atkins C.M., Selcher J.C., Petraitis J.J., Trzaskos J.M., Sweatt J.D. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1998;1:602–609. - PubMed
-
- Atkins C.M., Davare M.A., Oh M.C., Derkach V., Soderling T.R. Bidirectional regulation of cytoplasmic polyadenylation element-binding protein phosphorylation by Ca2+/calmodulin-dependent protein kinase II and protein phosphatase 1 during hippocampal long-term potentiation. J. Neurosci. 2005;25:5604–5610. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
