Multiple pathways for sorting mitochondrial precursor proteins
- PMID: 18174896
- PMCID: PMC2246611
- DOI: 10.1038/sj.embor.7401126
Multiple pathways for sorting mitochondrial precursor proteins
Abstract
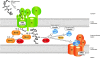
Mitochondria import hundreds of different precursor proteins from the cytosol. More than 50% of mitochondrial proteins do not use the classical import pathway that is guided by amino-terminal presequences, but instead contain different types of internal targeting signals. Recent studies have revealed an unexpected complexity of the mitochondrial protein import machinery and have led to the discovery of new transport pathways. Here, we review the versatility of mitochondrial protein import and its connection to mitochondrial morphology, redox regulation and energetics.
Figures
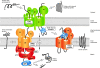






References
-
- Abe Y, Shodai T, Muto T, Mihara K, Torii H, Nishikawa S-I, Endo T, Kohda D (2000) Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell 100: 551–560 - PubMed
-
- Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatlidis K (2005) Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol 353: 937–944 - PubMed
-
- Becker L et al. (2005) Preprotein translocase of the outer mitochondrial membrane: reconstituted Tom40 forms a characteristic TOM pore. J Mol Biol 353: 1011–1020 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

