A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate
- PMID: 18175241
- PMCID: PMC2587364
- DOI: 10.1080/14767050701830480
A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate
Abstract
Introduction: Accumulating evidence suggests that an imbalance between pro-angiogenic (i.e., vascular endothelial growth factor (VEGF) and placental growth factor (PlGF)) and anti-angiogenic factors (i.e., soluble VEGF receptor-1 (sVEGFR-1, also referred to as sFlt1)) is involved in the pathophysiology of preeclampsia (PE). Endoglin is a protein that regulates the pro-angiogenic effects of transforming growth factor beta, and its soluble form has recently been implicated in the pathophysiology of PE. The objective of this study was to determine if changes in maternal plasma concentration of these angiogenic and anti-angiogenic factors differ prior to development of disease among patients with normal pregnancies and those destined to develop PE (preterm and term) or to deliver a small for gestational age (SGA) neonate.
Methods: This longitudinal nested case-control study included 144 singleton pregnancies in the following groups: (1) patients with uncomplicated pregnancies who delivered appropriate for gestational age (AGA) neonates (n = 46); (2) patients who delivered an SGA neonate but did not develop PE (n = 56); and (3) patients who developed PE (n = 42). Longitudinal samples were collected at each prenatal visit, scheduled at 4-week intervals from the first or early second trimester until delivery. Plasma concentrations of soluble endoglin (s-Eng), sVEGFR-1, and PlGF were determined by specific and sensitive ELISA.
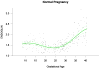
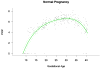
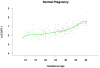
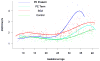
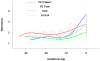
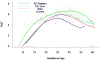
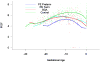
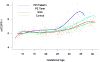
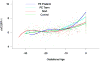
Results: (1) Patients destined to deliver an SGA neonate had higher plasma concentrations of s-Eng throughout gestation than those with normal pregnancies; (2) patients destined to develop preterm PE and term PE had significantly higher concentrations of s-Eng than those with normal pregnancies at 23 and 30 weeks, respectively (for preterm PE: p < 0.036 and for term PE: p = 0.002); (3) patients destined to develop PE (term or preterm) and those who delivered an SGA neonate had lower plasma concentrations of PlGF than those with a normal pregnancy throughout gestation, and the maternal plasma concentration of this analyte became detectable later among patients with pregnancy complications, compared to normal pregnant women; (4) there were no significant differences in the plasma concentrations of sVEGFR-1 between patients destined to deliver an SGA neonate and those with normal pregnancies; (5) patients destined to develop preterm and term PE had a significantly higher plasma concentration of sVEGFR-1 at 26 and 29 weeks of gestation than controls (p = 0.009 and p = 0.0199, respectively); and (6) there was no significant difference in the increment of sVEGFR-1 between control patients and those who delivered an SGA neonate (p = 0.147 at 25 weeks and p = 0.8285 at 40 weeks).
Conclusions: (1) Changes in the maternal plasma concentration of s-Eng, sVEGFR-1, and PlGF precede the clinical presentation of PE, but only changes in s-Eng and PlGF precede the delivery of an SGA neonate; and (2) differences in the profile of angiogenic and anti-angiogenic response to intrauterine insults may determine whether a patient will deliver an SGA neonate, develop PE, or both.
Figures
Comment in
-
The role of an 'anti-angiogenic state' in complications of pregnancy.J Matern Fetal Neonatal Med. 2008 Jan;21(1):3-7. doi: 10.1080/14767050701855081. J Matern Fetal Neonatal Med. 2008. PMID: 18175240 No abstract available.
References
-
- Campbell S, Diaz-Recasens J, Griffin DR, Cohen-Overbeek TE, Pearce JM, Willson K, Teague MJ. New doppler technique for assessing uteroplacental blood flow. Lancet. 1983;1:675–677. - PubMed
-
- Harrington KF, Campbell S, Bewley S, Bower S. Doppler velocimetry studies of the uterine artery in the early prediction of pre-eclampsia and intra-uterine growth retardation. Eur J Obstet Gynecol Reprod Biol. 1991;42(Suppl):S14–S20. - PubMed
-
- Bower S, Schuchter K, Campbell S. Doppler ultrasound screening as part of routine antenatal scanning: prediction of pre-eclampsia and intrauterine growth retardation. Br J Obstet Gynaecol. 1993;100:989–994. - PubMed
-
- Harrington K, Cooper D, Lees C, Hecher K, Campbell S. Doppler ultrasound of the uterine arteries: the importance of bilateral notching in the prediction of pre-eclampsia, placental abruption or delivery of a small-for-gestational-age baby. Ultrasound Obstet Gynecol. 1996;7:182–188. - PubMed
-
- Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179:1359–1375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
