Role of Lipids in Brain Injury and Diseases
- PMID: 18176634
- PMCID: PMC2174836
- DOI: 10.2217/17460875.2.4.403
Role of Lipids in Brain Injury and Diseases
Abstract
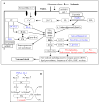
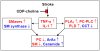
Lipid metabolism is of particular interest due to its high concentration in CNS. The importance of lipids in cell signaling and tissue physiology is demonstrated by many CNS disorders and injuries that involve deregulated metabolism. The long suffering lipid field is gaining reputation and respect as evidenced through the Center of Biomedical Research Excellence in Lipidomics and Pathobiology (COBRE), Lipid MAPS (Metabolites And Pathways Strategy) Consortium sponsored by NIH, European initiatives for decoding the lipids through genomic approaches, and Genomics of Lipid-associated Disorder (GOLD) project initiated by Austrian government. This review attempts to provide an overview of the lipid imbalances associated with neurological disorders (Alzheimer's, Parkinson's; Niemann-Pick; Multiple sclerosis, Huntington, amyotrophic lateral sclerosis, schizophrenia, bipolar disorders and epilepsy) and CNS injury (Stroke, traumatic brain injury; and spinal cord injury) and a few provocative thoughts. Lipidomic analyses along with RNA silencing will provide new insights into the role of lipid intermediates in cell signaling and hopefully open new avenues for prevention or treatment options.
Figures


References
-
-
Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4(7):594–610. ●● An excellent comprehensive review on various aspects of lipidomics and bioinformatics.
-
-
-
Fahy E, Subramaniam S, Brown HA, et al. A comprehensive classification system for lipids. J Lipid Res. 2005;46(5):839–862. ● A comprehensive classification of lipids with a common platform that is compatible with informatics requirements for the hard-core lipid community.
-
-
- Adibhatla RM, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40(3):376–387. - PubMed
-
- Jiang J, Borisenko GG, Osipov A, et al. Arachidonic acid-induced carbon-centered radicals and phospholipid peroxidation in cyclo-oxygenase-2-transfected PC12 cells. J Neurochem. 2004;90(5):1036–1049. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
