A clinical trial of combined use of rosiglitazone and 5-aminosalicylate for ulcerative colitis
- PMID: 18176972
- PMCID: PMC2673374
- DOI: 10.3748/wjg.14.114
A clinical trial of combined use of rosiglitazone and 5-aminosalicylate for ulcerative colitis
Abstract
Aim: To investigate the therapeutic effects of the combined use of rosiglitazone and aminosalicylate on mild or moderately active ulcerative colitis (UC).
Methods: According to the national guideline for diagnosis and treatment of inflammatory bowel disease (IBD) in China, patients with mild or moderately active UC in our hospital were selected from July to November, 2004. Patients with infectious colitis, amoebiasis, or cardiac, renal or hepatic failure and those who had received corticosteroid or immunosuppressant treatment within the last month were excluded. Following a quasi-randomization principle, patients were allocated alternatively into the treatment group (TG) with rosiglitazone 4 mg/d plus 5-ASA 2 g/d daily or the control group (CG) with 5-ASA 2 g/d alone, respectively, for 4 wk. Clinical changes were evaluated by Mayo scoring system and histological changes by Truelove-Richards' grading system at initial and final point of treatment.
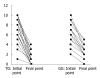
Results: Forty-two patients completed the trial, 21 each in TG and CG. The Mayo scores in TG at initial and final points were 5.87 (range: 4.29-7.43) and 1.86 (range: 1.03-2.69) and those in CG were 6.05 (range: 4.97-7.13) and 2.57 (range: 1.92-3.22) respectively. The decrements of Mayo scores were 4.01 in TG and 3.48 in CG, with a remission rate of 71.4% in TG and 57.1% in CG, respectively. Along with the improvement of disease activity index (DAI), the histological grade improvement was more significant in TG than in CG (P < 0.05).
Conclusion: Combined treatment with rosiglitazone and 5-ASA achieved better therapeutic effect than 5-ASA alone without any side effects. Rosiglitazone can alleviate colonic inflammation which hopefully becomes a novel agent for UC treatment.
Figures
References
-
- Lewis JD, Lichtenstein GR, Stein RB, Deren JJ, Judge TA, Fogt F, Furth EE, Demissie EJ, Hurd LB, Su CG, et al. An open-label trial of the PPAR-gamma ligand rosiglitazone for active ulcerative colitis. Am J Gastroenterol. 2001;96:3323–3328. - PubMed
-
- Ou-Yang Q, Pan GZ, Wen ZH, Wan XH, Hu RW, Lin SR, Hu PJ. Suggested guideline for the diagnosis and treatment of inflammatory bowel disease. Chin J Dig Dis. 2001;21:236–239.
-
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. - PubMed
-
- Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF, Auwerx J, Pettersson S, Desreumaux P. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265–1276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous