Combined Approaches to Site-Specific Modification of RNA
- PMID: 18177002
- PMCID: PMC2535795
- DOI: 10.1021/cb7002225
Combined Approaches to Site-Specific Modification of RNA
Abstract
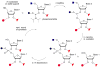
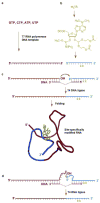
Both natural and unnatural modifications in RNA are of interest to biologists and chemists. More than 100 different analogues of the four standard RNA nucleosides have been identified in nature. Unnatural modifications are useful for structure and mechanistic studies of RNA. This Review highlights chemical, enzymatic, and combined (semisynthesis) approaches to generate site specifically modified RNAs. The availability of these methods for site-specific modifications of RNAs of all sizes is important in order to study the relationships between RNA chemical composition, structure, and function.
Figures

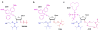
Similar articles
-
Chemical and Conformational Diversity of Modified Nucleosides Affects tRNA Structure and Function.Biomolecules. 2017 Mar 16;7(1):29. doi: 10.3390/biom7010029. Biomolecules. 2017. PMID: 28300792 Free PMC article. Review.
-
Amide-Modified RNA: Using Protein Backbone to Modulate Function of Short Interfering RNAs.Acc Chem Res. 2020 Sep 15;53(9):1782-1790. doi: 10.1021/acs.accounts.0c00249. Epub 2020 Jul 13. Acc Chem Res. 2020. PMID: 32658452 Free PMC article.
-
The occurrence order and cross-talk of different tRNA modifications.Sci China Life Sci. 2021 Sep;64(9):1423-1436. doi: 10.1007/s11427-020-1906-4. Epub 2021 Apr 19. Sci China Life Sci. 2021. PMID: 33881742 Review.
-
Re-Engineering RNA Molecules into Therapeutic Agents.Acc Chem Res. 2019 Apr 16;52(4):1036-1047. doi: 10.1021/acs.accounts.8b00650. Epub 2019 Mar 26. Acc Chem Res. 2019. PMID: 30912917
-
Probing Nascent RNA with Metabolic Incorporation of Modified Nucleosides.Acc Chem Res. 2022 Sep 20;55(18):2647-2659. doi: 10.1021/acs.accounts.2c00347. Epub 2022 Sep 8. Acc Chem Res. 2022. PMID: 36073807
Cited by
-
Site-specific RNA modification via initiation of in vitro transcription reactions with m6A and isomorphic emissive adenosine analogs.RSC Chem Biol. 2024 Mar 27;5(5):454-458. doi: 10.1039/d4cb00045e. eCollection 2024 May 8. RSC Chem Biol. 2024. PMID: 38725913 Free PMC article.
-
Probing the stabilizing effects of modified nucleotides in the bacterial decoding region of 16S ribosomal RNA.Bioorg Med Chem. 2013 May 15;21(10):2720-6. doi: 10.1016/j.bmc.2013.03.010. Epub 2013 Mar 21. Bioorg Med Chem. 2013. PMID: 23566761 Free PMC article.
-
Site-Selective Modification and Labeling of Native RNA.Chemistry. 2025 Feb 25;31(12):e202404244. doi: 10.1002/chem.202404244. Epub 2025 Feb 9. Chemistry. 2025. PMID: 39865772 Free PMC article. Review.
-
Polymerase-Mediated Site-Specific Incorporation of a Synthetic Fluorescent Isomorphic G Surrogate into RNA.Angew Chem Int Ed Engl. 2017 Jan 24;56(5):1303-1307. doi: 10.1002/anie.201609327. Epub 2016 Dec 21. Angew Chem Int Ed Engl. 2017. PMID: 28000329 Free PMC article.
-
A new usage of functionalized oligodeoxynucleotide probe for site-specific modification of a guanine base within RNA.Nucleic Acids Res. 2010 Mar;38(5):1760-6. doi: 10.1093/nar/gkp930. Epub 2010 Jan 31. Nucleic Acids Res. 2010. PMID: 20123727 Free PMC article.
References
-
- Grosjean H. Modification and editing of RNA: historical overview and important facts to remember. In: Grosjean H, editor. Fine-Tuning of RNA Functions by Modification and Editing. 1. Springer-Verlag; New York: 2005. pp. 1–16.
-
- Ye K. H/ACA guide RNAs, proteins and complexes. Curr Op Struct Biol. 2007;17:287–292. - PubMed
-
- Gallo M, Montserrat JM, Iribarren AM. Design and applications of modified oligonucleotides. Braz J Med Biol Res. 2003;36:143–151. - PubMed
-
- Zimmermann RA, Gait MJ, Moore MJ. Incorporation of modified nucleotides into RNA for studies on RNA structure, function, and intermolecular interactions. In: Grosjean H, Benne R, editors. Modification and Editing of RNA. ASM Press; Washington, DC: 1998. pp. 59–84.
Grants and funding
LinkOut - more resources
Full Text Sources

