Inferior rabies vaccine quality and low immunization coverage in dogs ( Canis familiaris) in China
- PMID: 18177524
- PMCID: PMC2870756
- DOI: 10.1017/S0950268807000131
Inferior rabies vaccine quality and low immunization coverage in dogs ( Canis familiaris) in China
Abstract
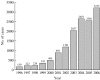
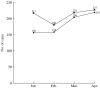
Human rabies in China continues to increase exponentially, largely due to an inadequate veterinary infrastructure and poor vaccine coverage of naive dogs. We performed an epidemiological survey of rabies both in humans and animals, examined vaccine quality for animal use, evaluated the vaccination coverage in dogs, and checked the dog samples for the presence of rabies virus. The lack of surveillance in dog rabies, together with the low immunization coverage (up to 2.8% in rural areas) and the high percentage of rabies virus prevalence (up to 6.4%) in dogs, suggests that the dog population is a continual threat for rabies transmission from dogs to humans in China. Results also indicated that the quality of rabies vaccines for animal use did not satisfy all of the requirements for an efficacious vaccine capable of fully eliminating rabies. These data suggest that the factors noted above are highly correlated with the high incidence of human rabies in China.
Figures
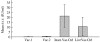

References
-
- Fooks AR. Rabies in Europe – editorial. Eurosurveillance. 2005;10:1–4. - PubMed
-
- Ministry of Health of the People's Republic of China. http://www.moh.gov.cn http://www.moh.gov.cn
-
- World Health Organization 2005. . WHO Expert Consultation on Rabies. First Report WHO Technical Report Series, No. 931 .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

