Identification of human UDP-glucuronosyltransferases catalyzing hepatic 1alpha,25-dihydroxyvitamin D3 conjugation
- PMID: 18177842
- PMCID: PMC2664830
- DOI: 10.1016/j.bcp.2007.11.008
Identification of human UDP-glucuronosyltransferases catalyzing hepatic 1alpha,25-dihydroxyvitamin D3 conjugation
Abstract
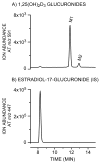
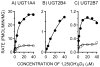
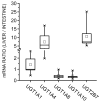
The biological effects of 1alpha,25-dihydroxyvitamin D3 (1,25(OH)2D3) are terminated primarily by P450-dependent hydroxylation reactions. However, the hormone is also conjugated in the liver and a metabolite, presumably a glucuronide, undergoes enterohepatic cycling. In this study, the identity of human enzymes capable of catalyzing the 1,25(OH)2D3 glucuronidation reaction was investigated in order to better understand environmental and endogenous factors affecting the disposition and biological effects of vitamin D3. Among 12 different UGT isozymes tested, only UGT1A4 >> 2B4 and 2B7 supported the reaction. Two different 1,25(OH)2D3 monoglucuronide metabolites were generated by recombinant UGT1A4 and human liver microsomes. The most abundant product was identified by mass spectral and NMR analyses as the 25-O-glucuronide isomer. The formation of 25-O-glucuronide by UGT1A4 Supersomes and human liver microsomes followed simple hyperbolic kinetics, yielding respective Km and Vmax values of 7.3 and 11.2 microM and 33.7 +/- 1.4 and 32.9 +/- 1.9 pmol/min/mg protein. The calculated intrinsic 25-O-glucuronide M1 formation clearance for UGT1A4 was 14-fold higher than the next best isozyme, UGT2B7. There was only limited (four-fold) inter-liver variability in the 25-O-glucuronidation rate, but it was highly correlated with the relative rate of formation of the second, minor metabolite. In addition, formation of both metabolites was inhibited >80% by the selective UGT1A4 inhibitor, hecogenin. If enterohepatic recycling of 1,25(OH)2D3 represents a significant component of intestinal and systemic 1,25(OH)2D3 disposition, formation of monoglucuronides by hepatic UGT1A4 constitutes an important initial step.
Figures



References
-
- Lown KS, Kolars JC, Thummel KE, Barnett JL, Kunze KL, Wrighton SA, et al. Interpatient heterogeneity in expression of CYP3A4 and CYP3A5 in small bowel: lack of prediction by the erythromycin breath test. Drug Metab Dispos. 1994;22:947–55. - PubMed
-
- von Richter O, Burk O, Fromm MF, Thon KP, Eichelbaum M, Kivisto KT. Cytochrome P450 3A4 and P-glycoprotein expression in human small intestinal enterocytes and hepatocytes: a comparative analysis in paired tissue specimens. Clin Pharmacol Ther. 2004;75:172–83. - PubMed
-
- Schmiedlin-Ren P, Thummel KE, Fisher JM, Paine MF, Lown KS, Watkins PB. Expression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1α,25-dihydroxyvitamin D3. Mol Pharmacol. 1997;51:741–54. - PubMed
-
- Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, et al. Transcriptional control of intestinal cytochrome P-4503A by 1α,25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60:1399–406. - PubMed
-
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

