Structural and functional characterization of a new recombinant histidine-tagged acyl coenzyme A binding protein (ACBP) from mouse
- PMID: 18178100
- PMCID: PMC2367699
- DOI: 10.1016/j.pep.2007.11.010
Structural and functional characterization of a new recombinant histidine-tagged acyl coenzyme A binding protein (ACBP) from mouse
Abstract
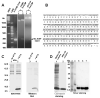
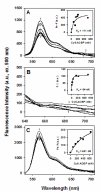
Acyl coenzyme A binding protein (ACBP) has been proposed to transport fatty acyl CoAs intracellularly, facilitating their metabolism. In this study, a new mouse recombinant ACBP was produced by insertion of a histidine (his) tag at the C-terminus to allow efficient purification by Ni-affinity chromatography. The his-tag was inserted at the C-terminus since ACBP is a small molecular size (10 kDa) protein whose structure and activity are sensitive to amino acid substitutions in the N-terminus. The his-tag had no or little effect on ACBP structure or ligand binding affinity and specificity. His-ACBP bound the naturally occurring fluorescent cis-parinaroyl-CoA with very high affinity (K(d)=2.15 nM), but exhibited no affinity for non-esterified cis-parinaric acid. To determine if the presence of the C-terminal his-tag altered ACBP interactions with other proteins, direct binding to hepatocyte nuclear factor-4alpha (HNF-4alpha), a nuclear receptor regulating transcription of genes involved in lipid metabolism, was examined. His-ACBP and HNF-4alpha were labeled with Cy5 and Cy3, respectively, and direct interaction was determined by a novel fluorescence resonance energy transfer (FRET) binding assay. FRET analysis showed that his-ACBP directly interacted with HNF-4alpha (intermolecular distance of 73 A) at high affinity (K(d)=64-111 nM) similar to native ACBP. The his-tag also had no effect on ACBPs ability to interact with and stimulate microsomal enzymes utilizing or forming fatty acyl CoA. Thus, C-terminal his-tagged-ACBP maintained very similar structural and functional features of the untagged native protein and can be used in further in vitro experiments that require pure recombinant ACBP.
Figures



Similar articles
-
Membrane charge and curvature determine interaction with acyl-CoA binding protein (ACBP) and fatty acyl-CoA targeting.Biochemistry. 2002 Aug 20;41(33):10540-53. doi: 10.1021/bi0259498. Biochemistry. 2002. PMID: 12173941
-
Acyl-coenzyme A binding protein expression alters liver fatty acyl-coenzyme A metabolism.Biochemistry. 2005 Aug 2;44(30):10282-97. doi: 10.1021/bi0477891. Biochemistry. 2005. PMID: 16042405
-
Role of regulatory F-domain in hepatocyte nuclear factor-4alpha ligand specificity.J Biol Chem. 2005 Apr 29;280(17):16714-27. doi: 10.1074/jbc.M405906200. Epub 2005 Feb 28. J Biol Chem. 2005. PMID: 15741159
-
Acyl-CoA-binding protein (ACBP) and its relation to fatty acid-binding protein (FABP): an overview.Mol Cell Biochem. 1990 Oct 15-Nov 8;98(1-2):217-23. doi: 10.1007/BF00231387. Mol Cell Biochem. 1990. PMID: 2266962 Review.
-
Involvement of low molecular mass soluble acyl-CoA-binding protein in seed oil biosynthesis.N Biotechnol. 2011 Feb 28;28(2):97-109. doi: 10.1016/j.nbt.2010.09.011. Epub 2010 Oct 8. N Biotechnol. 2011. PMID: 20933624 Review.
Cited by
-
L-FABP directly interacts with PPARalpha in cultured primary hepatocytes.J Lipid Res. 2009 Aug;50(8):1663-75. doi: 10.1194/jlr.M900058-JLR200. Epub 2009 Mar 16. J Lipid Res. 2009. PMID: 19289416 Free PMC article.
-
Glucose regulates fatty acid binding protein interaction with lipids and peroxisome proliferator-activated receptor α.J Lipid Res. 2010 Nov;51(11):3103-16. doi: 10.1194/jlr.M005041. Epub 2010 Jul 13. J Lipid Res. 2010. PMID: 20628144 Free PMC article.
-
Inhibitors of Fatty Acid Synthesis Induce PPAR α -Regulated Fatty Acid β -Oxidative Genes: Synergistic Roles of L-FABP and Glucose.PPAR Res. 2013;2013:865604. doi: 10.1155/2013/865604. Epub 2013 Feb 26. PPAR Res. 2013. PMID: 23533380 Free PMC article.
-
Expression of acyl-CoA-binding protein 5 from Rhodnius prolixus and its inhibition by RNA interference.PLoS One. 2020 Jan 14;15(1):e0227685. doi: 10.1371/journal.pone.0227685. eCollection 2020. PLoS One. 2020. PMID: 31935250 Free PMC article.
-
Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription.Lipids. 2008 Jan;43(1):1-17. doi: 10.1007/s11745-007-3111-z. Epub 2007 Sep 19. Lipids. 2008. PMID: 17882463 Review.
References
-
- Abo-Hashema KAH, Cake MH, Lukas MA, Knudsen J. The interaction of acyl CoA with acyl CoA binding protein and carnitine palmitoyltransferase I. Int. J. Biochem. & Cell Biol. 2001;33:807–815. - PubMed
-
- Berde CB, Hudson BS, Simoni RD, Sklar LA. Human serum albumin. Spectroscopic studies of binding and proximity relationships for fatty acids adn bilirubin. J. Biol. Chem. 1979;254:391–400. - PubMed
-
- Bhuiyan AKMJ, Pande SV. Carnitine palmitoyltransferase activies: effects of serum albumin, ACBP, and FABP. Mol. Cell. Biochem. 1994;139:109–116. - PubMed
-
- Chao H, Martin G, Russell WK, Waghela SD, Russell DH, Schroeder F, Kier AB. Membrane charge and curvature determine interaction with acyl CoA binding protein (ACBP) and fatty acyl CoA targeting. Biochemistry. 2002;41:10540–10553. - PubMed
-
- Chao H, Zhou M, McIntosh A, Schroeder F, Kier AB. Acyl CoA binding protein and cholesterol differentially alter fatty acyl CoA utilization by microsomal acyl CoA: cholesterol transferase. J. Lipid Res. 2003;44:72–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

