Peptide mimic of the HIV envelope gp120-gp41 interface
- PMID: 18178220
- PMCID: PMC2265733
- DOI: 10.1016/j.jmb.2007.12.001
Peptide mimic of the HIV envelope gp120-gp41 interface
Abstract
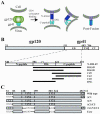
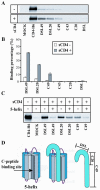
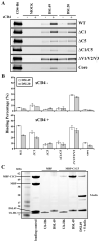
The human immunodeficiency virus envelope glycoprotein (Env) is composed of surface (gp120) and transmembrane (gp41) subunits, which are noncovalently associated on the viral surface. Human immunodeficiency virus Env mediates viral entry after undergoing a complex series of conformational changes induced by interaction with cellular CD4 and a chemokine coreceptor. These changes propagate from gp120 to gp41 via the gp120-gp41 interface, ultimately exposing gp41 and allowing it to form the trimer-of-hairpins structure that provides the driving force for membrane fusion. Key unresolved questions about the gp120-gp41 interface include the specific regions of gp41 and gp120 involved, the mechanism by which receptor and coreceptor-binding-induced conformational changes in gp120 are communicated to gp41, how trimer-of-hairpins formation is prevented in the prefusogenic gp120-gp41 complex, and, ultimately, the structure of the prefusion gp120-gp41 complex. Here, we develop a biochemical model system that mimics a key portion of the gp120-gp41 interface in the prefusogenic state. We find that a gp41 fragment containing the disulfide bond loop and C-peptide region binds primarily to the gp120 C5 region and that this interaction is incompatible with trimer-of-hairpins formation. Based on these data, we propose that in prefusogenic Env, gp120 sequesters the gp41 C-peptide region away from the N-trimer region, preventing trimer-of-hairpins formation until coreceptor binding disrupts this interface. This model system is a valuable tool for studying the gp120-gp41 complex, conformational changes induced by CD4 and coreceptor binding, and the mechanism of membrane fusion.
Figures

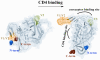
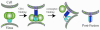
References
-
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–8. - PubMed
-
- Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. - PubMed
-
- Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–83. - PubMed
-
- Chen B, Vogan EM, Gong H, Skehel JJ, Wiley DC, Harrison SC. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature. 2005;433:834–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

