Characterization of the interactions between fluoroquinolone antibiotics and lipids: a multitechnique approach
- PMID: 18178657
- PMCID: PMC2275711
- DOI: 10.1529/biophysj.107.114843
Characterization of the interactions between fluoroquinolone antibiotics and lipids: a multitechnique approach
Abstract
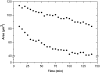
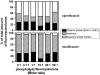
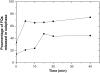
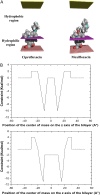
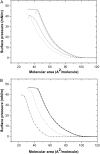
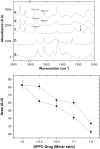
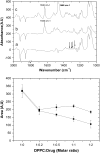
Probing drug/lipid interactions at the molecular level represents an important challenge in pharmaceutical research and membrane biophysics. Previous studies showed differences in accumulation and intracellular activity between two fluoroquinolones, ciprofloxacin and moxifloxacin, that may actually result from their differential susceptibility to efflux by the ciprofloxacin transporter. In view of the critical role of lipids for the drug cellular uptake and differences observed for the two closely related fluoroquinolones, we investigated the interactions of these two antibiotics with lipids, using an array of complementary techniques. Moxifloxacin induced, to a greater extent than ciprofloxacin, an erosion of the DPPC domains in the DOPC fluid phase (atomic force microscopy) and a shift of the surface pressure-area isotherms of DOPC/DPPC/fluoroquinolone monolayer toward lower area per molecule (Langmuir studies). These effects are related to a lower propensity of moxifloxacin to be released from lipid to aqueous phase (determined by phase transfer studies and conformational analysis) and a marked decrease of all-trans conformation of acyl-lipid chains of DPPC (determined by ATR-FTIR) without increase of lipid disorder and change in the tilt between the normal and the germanium surface (also determined by ATR-FTIR). All together, differences of ciprofloxacin as compared to moxifloxacin in their interactions with lipids could explain differences in their cellular accumulation and susceptibility to efflux transporters.
Figures
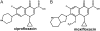

References
-
- Michot, J. M., C. Seral, F. Van Bambeke, M. P. Mingeot-Leclercq, and P. Tulkens. 2005. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob. Agents Chemother. 49:2429–2437. - PMC - PubMed
-
- Wong, J. P., H. Yang, K. L. Blasetti, G. Schnell, J. Conley, and L. N. Schofield. 2003. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J. Control. Release. 92:265–273. - PubMed
-
- Rolston, K. V., D. Yadegarynia, D. P. Kontoyiannis, I. I. Raad, and D. H. Ho. 2006. The spectrum of Gram-positive bloodstream infections in patients with hematologic malignancies, and the in vitro activity of various quinolones against Gram-positive bacteria isolated from cancer patients. Int. J. Infect. Dis. 10:223–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

