Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes
- PMID: 18178659
- PMCID: PMC2267117
- DOI: 10.1529/biophysj.107.114090
Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes
Abstract
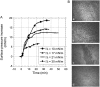
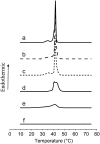
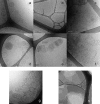
Fengycin is a biologically active lipopeptide produced by several Bacillus subtilis strains. The lipopeptide is known to develop antifungal activity against filamentous fungi and to have hemolytic activity 40-fold lower than that of surfactin, another lipopeptide produced by B. subtilis. The aim of this work is to use complementary biophysical techniques to reveal the mechanism of membrane perturbation by fengycin. These include: 1), the Langmuir trough technique in combination with Brewster angle microscopy to study the lipopeptide penetration into monolayers; 2), ellipsometry to investigate the adsorption of fengycin onto supported lipid bilayers; 3), differential scanning calorimetry to determine the thermotropic properties of lipid bilayers in the presence of fengycin; and 4), cryogenic transmission electron microscopy, which provides information on the structural organization of the lipid/lipopeptide system. From these experiments, the mechanism of fengycin action appears to be based on a two-state transition controlled by the lipopeptide concentration. One state is the monomeric, not deeply anchored and nonperturbing lipopeptide, and the other state is a buried, aggregated form, which is responsible for membrane leakage and bioactivity. The mechanism, thus, appears to be driven mainly by the physicochemical properties of the lipopeptide, i.e., its amphiphilic character and affinity for lipid bilayers.
Figures






Similar articles
-
Fengycin interaction with lipid monolayers at the air-aqueous interface-implications for the effect of fengycin on biological membranes.J Colloid Interface Sci. 2005 Mar 15;283(2):358-65. doi: 10.1016/j.jcis.2004.09.036. J Colloid Interface Sci. 2005. PMID: 15721905
-
Leakage and lysis of lipid membranes induced by the lipopeptide surfactin.Eur Biophys J. 2007 Apr;36(4-5):305-14. doi: 10.1007/s00249-006-0091-5. Epub 2006 Oct 19. Eur Biophys J. 2007. PMID: 17051366
-
Antimycotic activity of fengycin C biosurfactant and its interaction with phosphatidylcholine model membranes.Colloids Surf B Biointerfaces. 2017 Aug 1;156:114-122. doi: 10.1016/j.colsurfb.2017.05.021. Epub 2017 May 10. Colloids Surf B Biointerfaces. 2017. PMID: 28527355
-
Lipopeptide surfactants: Production, recovery and pore forming capacity.Peptides. 2015 Sep;71:100-12. doi: 10.1016/j.peptides.2015.07.006. Epub 2015 Jul 17. Peptides. 2015. PMID: 26189973 Review.
-
Key elements and regulation strategies of NRPSs for biosynthesis of lipopeptides by Bacillus.Appl Microbiol Biotechnol. 2020 Oct;104(19):8077-8087. doi: 10.1007/s00253-020-10801-x. Epub 2020 Aug 19. Appl Microbiol Biotechnol. 2020. PMID: 32813066 Review.
Cited by
-
Opportunistic Challenges of Computer-aided Drug Discovery of Lipopeptides: New Insights for Large Molecule Therapeutics.Avicenna J Med Biotechnol. 2023 Jan-Mar;15(1):3-13. doi: 10.18502/ajmb.v15i1.11419. Avicenna J Med Biotechnol. 2023. PMID: 36789119 Free PMC article. Review.
-
Role of Lipid Composition, Physicochemical Interactions, and Membrane Mechanics in the Molecular Actions of Microbial Cyclic Lipopeptides.J Membr Biol. 2019 Jun;252(2-3):131-157. doi: 10.1007/s00232-019-00067-4. Epub 2019 May 16. J Membr Biol. 2019. PMID: 31098678 Review.
-
Bacillus licheniformis: A Producer of Antimicrobial Substances, including Antimycobacterials, Which Are Feasible for Medical Applications.Pharmaceutics. 2023 Jul 5;15(7):1893. doi: 10.3390/pharmaceutics15071893. Pharmaceutics. 2023. PMID: 37514078 Free PMC article. Review.
-
Cellulose-dependent expression and antibacterial characteristics of surfactin from Bacillus subtilis HH2 isolated from the giant panda.PLoS One. 2018 Jan 31;13(1):e0191991. doi: 10.1371/journal.pone.0191991. eCollection 2018. PLoS One. 2018. PMID: 29385201 Free PMC article.
-
Biocombinatorial Synthesis of Novel Lipopeptides by COM Domain-Mediated Reprogramming of the Plipastatin NRPS Complex.Front Microbiol. 2016 Nov 17;7:1801. doi: 10.3389/fmicb.2016.01801. eCollection 2016. Front Microbiol. 2016. PMID: 27909427 Free PMC article.
References
-
- Vanittanakom, N., W. Loeffler, U. Koch, and G. Jung. 1986. Fengycin—a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J. Antibiot. (Tokyo). 39:888–901. - PubMed
-
- Jacques, P., C. Hbid, J. Destain, H. Razafindralambo, M. Paquot, E. De Pauw, and P. Thonart. 1999. Optimization of biosurfactant lipopeptide production from Bacillus subtilis S499 by Plackett-Burman design. Appl. Biochem. Biotechnol. 77–79:223–233.
-
- Hbid, C. 1996. Contribution à l'étude de la relation entre la structure des lipopeptides de B. subtilis et leurs activités hémolytique et antifongique. PhD thesis. Université de Liège, Belgium.
-
- Schneider, J., K. Taraz, H. Budzikiewicz, M. Deleu, P. Thonart, and P. Jacques. 1999. The structure of two fengycins from Bacillus subtilis S499. Z. Naturforsch [C]. 54:859–866. - PubMed
-
- Deleu, M., M. Paquot, and T. Nylander. 2005. Fengycin interaction with lipid monolayers at the air-aqueous interface—implications for the effect of fengycin on biological membranes. J. Colloid Interface Sci. 283:358–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

